Overview
- Peptide (C)GKVDGTSHVTGKG, corresponding to amino acid residues 40 - 52 of human PAR2/F2RL1 (Accession P55085). Extracellular, N-terminus.
- Human PANC-1 pancreatic carcinoma, THP-1 monocytic leukemia, Jurkat T-cell, and MCF-7 breast adenocarcinoma cell lysates (1:200-1:1000).
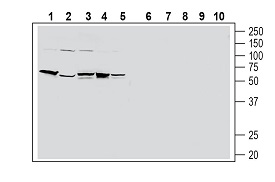 Western blot analysis of human HT-29 colon adenocarcinoma cell line lysate (lanes 1 and 6), human PANC-1 pancreatic carcinoma cell line lysate (lanes 2 and 7), human THP-1 monocytic leukemia cell line lysate (lanes 3 and 8), human Jurkat T-cell leukemia cell line lysate (lanes 4 and 9) and human MCF-7 breast adenocarcinoma cell line lysate (lanes 5 and 10):1-5. Anti-Human PAR2/F2RL1 (extracellular) Antibody (#APR-035), (1:200).
Western blot analysis of human HT-29 colon adenocarcinoma cell line lysate (lanes 1 and 6), human PANC-1 pancreatic carcinoma cell line lysate (lanes 2 and 7), human THP-1 monocytic leukemia cell line lysate (lanes 3 and 8), human Jurkat T-cell leukemia cell line lysate (lanes 4 and 9) and human MCF-7 breast adenocarcinoma cell line lysate (lanes 5 and 10):1-5. Anti-Human PAR2/F2RL1 (extracellular) Antibody (#APR-035), (1:200).
6-10. Anti-Human PAR2/F2RL1 (extracellular) Antibody, preincubated with Human PAR2/F2RL1 (extracellular) Blocking Peptide (#BLP-PR035) (#BLP-PR035).
- Human THP-1 monocytic leukemia cells (2.5 μg).
Protease-activated receptor 2 (PAR-2) belongs to a family of four G protein-coupled receptors (PAR1-4) that are activated as a result of proteolytic cleavage by certain serine proteases, hence their name. In this novel modality of activation, a specific protease cleaves the PAR receptor within a defined sequence in its extracellular N-terminal domain. This results in the creation of a new N-terminal tethered ligand, which subsequently binds to a site in the second extracellular loop of the same receptor. This binding results in the coupling of the receptor to G proteins and in the activation of several signal transduction pathways1-3.
Different PARs are activated by different proteases. Hence, PAR-1 is activated by thrombin, as are PAR-3 and PAR-4.1-3 PAR-2 is the only PAR family receptor that is activated by trypsin and not by thrombin. PAR-2 can be also cleaved and activated by other proteases such as tryptase, membrane-type serine protease 1, protease 3, and others1-3.
The intramolecular nature of PAR activation and the continuous presence of the tethered ligand that cannot diffuse away imply the existence of several mechanisms for the rapid termination of PAR signaling. Indeed, following receptor activation, there is rapid phosphorylation of the C-terminal end of the receptor, followed by receptor internalization and degradation. In addition, several proteases can cleave away the tethered ligand, thereby “disarming” the PAR1-3.
PAR-2-mediated intracellular signaling has not been clearly elucidated, but activators of PAR-2 induce generation of IP3 and mobilization of intracellular Ca2+. Activation of the ERK1/2 and NF-kB intracellular pathways following PAR-2 activation has been also described1-3.
Tissue distribution of PAR-2 is wide with the highest expression levels found in pancreas, small intestine, liver and kidney. In addition, PAR-2 expression was observed in dorsal root ganglion neurons, smooth muscle cells and endothelial cells.
The physiological role of PAR-2 is not clearly understood, however studies using PAR-2 knockout mice have suggested roles in the cardiovascular, pulmonary and gastrointestinal systems. Several studies suggest that PAR-2 plays a central role in inflammatory diseases and nociceptive pain transduction; hence PAR-2 blockers have been proposed to be useful in the therapeutic control of inflammation and pain1-3.
