Overview
- Peptide (C)RLGFLGSNTPHENH, corresponding to amino acid residues 2683-2696 of rat IP3R2 (Accession P29995). Intracellular, C-terminus.
- Rat brain membrane and rat basophilic leukemia cell lysate (1:200-1:1000).
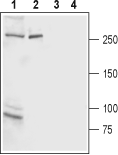 Western blot analysis of rat brain membrane (lanes 1 and 3) and rat basophilic leukemia (RBL) cell line lysate (lanes 2 and 4):1,2. Anti-IP3 Receptor-2 (ITPR2) Antibody (#ACC-116), (1:200).
Western blot analysis of rat brain membrane (lanes 1 and 3) and rat basophilic leukemia (RBL) cell line lysate (lanes 2 and 4):1,2. Anti-IP3 Receptor-2 (ITPR2) Antibody (#ACC-116), (1:200).
3,4. Anti-IP3 Receptor-2 (ITPR2) Antibody, preincubated with IP3 Receptor-2/ITPR2 Blocking Peptide (#BLP-CC116).
- Rat spinal cord sections.
Inositol 1,4,5-trisphosphate receptors (IP3R) are intracellular Ca2+ release channels located on the endoplasmic reticulum (ER) and mediate Ca2+ mobilization from the ER to the cytoplasm in response to the binding of the second messenger, inositol 1,4,5-trisphosphate (IP3)1. IP3-induced Ca2+ release is triggered by various external stimuli, and most non-excitable cells use this mechanism as the primary Ca2+ signaling pathway. IP3Rs are therefore thought to have important physiological roles in various cell types and tissues2.
Three subtypes of IP3Rs, derived from three distinct genes, have been identified in mammals3. All three receptors have six transmembrane domains and a pore region between TM5 and TM6. The N-terminus as well as the C-terminus are cytoplasmic. Each IP3R consists of an N-terminal ligand binding domain (LBD) and a C-terminal domain which is linked by a long regulatory domain. The C-terminus is constitutively active, suggesting that the regulatory domain is required to maintain the suppression of channel activity4.
Type 2 IP3R (IP3R2) is expressed in various tissues and cell lines. IP3R2 mRNA is localized in the intralobular duct cells of the submandibular gland, the urinary tubule cells of the kidney, the epithelial cells of epididymal ducts and the follicular granulosa cells of the ovary5. IP3R2 is active during osteoclast differentiation, and its absence causes a partial defect in osteoclastic differentiation.
