Overview
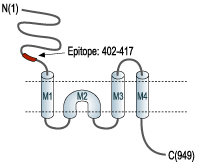
- Peptide (C)KASGEVSKHLYKVWKK, corresponding to amino acid residues 402-417 of rat kainate receptor GluK1 (Accession P22756). Extracellular, N-terminus.
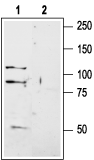 Western blot analysis of rat brain membranes:1. Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:200).
Western blot analysis of rat brain membranes:1. Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:200).
2. Anti-GRIK1 (GluK1) (extracellular) Antibody, preincubated with GRIK1/GluK1 (extracellular) Blocking Peptide (#BLP-GC008).
 Expression of GluK1 in rat cerebellumImmunohistochemical staining of perfusion-fixed frozen rat brain sections using Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:400), followed by donkey anti rabbit-Cy3 antibody. GluK1 staining (red) appears in the granule layer and in the baskets under Purkinje cells (arrows). Nuclei were labeled with DAPI and appear in blue.
Expression of GluK1 in rat cerebellumImmunohistochemical staining of perfusion-fixed frozen rat brain sections using Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:400), followed by donkey anti rabbit-Cy3 antibody. GluK1 staining (red) appears in the granule layer and in the baskets under Purkinje cells (arrows). Nuclei were labeled with DAPI and appear in blue.
 Expression of kainate receptor GluK1 in rat DRG neuronsCell surface detection of kainate receptor GluK1 in living rat dorsal root ganglion (DRG). A. Extracellular staining of cells using Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:50) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody (red). B. Live view of the same field.
Expression of kainate receptor GluK1 in rat DRG neuronsCell surface detection of kainate receptor GluK1 in living rat dorsal root ganglion (DRG). A. Extracellular staining of cells using Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), (1:50) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody (red). B. Live view of the same field.
- Lerma, J. et al. (2001) Physiol. Rev. 81, 971.
- Dingledine, R. et al. (1999) Pharmacol. Rev. 51, 7.
L-Glutamate, the major excitatory neurotransmitter in the central nervous system (CNS), operates through several receptors that are categorized as ionotropic (ligand-gated cation channels) or metabotropic (G-protein-coupled receptors).
The ligand-gated ion channel family consists of fifteen members that have been subdivided into three families based upon their pharmacological profile: the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-preferring receptors, the N-methyl-D-aspartate (NMDA)-preferring receptors, and the kainate-preferring receptors.
The kainate receptor subfamily consists of five members that have been further subdivided into two classes based upon structural homology and functional characteristics. GluR5, GluR6, and GluR7receptor subunits share a high degree of homology and are able to form functional channels when expressed in heterologous systems. The KA-1 and KA-2 receptors are unable to form functional channels on their own, but when coexpressed with GluR5-7 receptor subunits, they form channels with high affinity for kainate.1,2
Like AMPA receptors, the functional unit of endogenous kainate receptors is believed to be a tetramer, which can be either homomeric or heteromeric. Kainate receptors GluR5 and GluR6 (but not GluR7, KA-1, or KA-2) can undergo RNA editing; as in the AMPA receptor GluR2, a glutamine (Q) residue in the channel pore is edited to encode arginine (R) in the mature protein. Substitution of Q with R modulates the properties of the channel, producing channels with reduced single channel conductance and lower permeability to Ca2+.1,2
GluR5 is highly expressed in dorsal root ganglion (DRG) neurons of the peripheral nervous system (PNS) as well as in several structures of the CNS including the amygdala, the hipoccampus, and Purkinje cells of the cerebellum.
GluR5 has been implicated in the modulation of synaptic transmission and synaptic plasticity in both the CNS and PNS, but the exact physiological function of GluR5 is not well understood. Nevertheless, an involvement in persistent pain and some types of learning has been proposed.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer Anti-GRIK1 (GluK1) (extracellular) Antibody (#AGC-008), a highly specific antibody directed against the extracellular N-terminus domain of the rat kainate receptor GluK1. The antibody can be used in western blot and immunofluorescence applications.
Applications
Citations
- Mouse DRG sections (1:500).
Mitchell, R. et al. (2019) Mol. Neurobiol. 56, 5917.
- Mouse DRG sections (1:500).
Mitchell, R. et al. (2019) Mol. Neurobiol. 56, 5917.
- Marrocco, J. et al. (2012) J. Neurosci. 32, 17143.
