Overview
- Peptide DTSGHFHDSGVGDLDEDPKC, corresponding to amino acid residues 2-21 of human KCNN3 (Accession Q9UGI6). Intracellular, N-terminus.
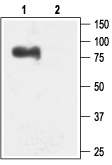 Western blot analysis of rat brain membranes:1. Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025), (1:200).
Western blot analysis of rat brain membranes:1. Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025), (1:200).
2. Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody, preincubated with KCNN3/KCa2.3 (N-term) Blocking Peptide (#BLP-PC025).- Mouse and human uterine tissue (1:100) (Pierce, S.L. and England, S.K. (2010) Am. J. Physiol. 299, E640.).
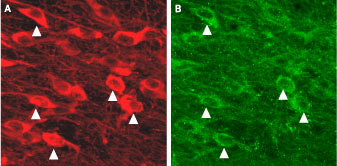 Expression of KCa2.3 (SK3) in mouse dopaminergic neuronsImmunohistochemical staining of mouse dopaminergic neurons using Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025). A. KCa2.3 is detected in substantia nigra pars compacta. B. Tyrosine hydroxylase staining shows dopaminergic neurons. Triangles point at cells with co-localization.
Expression of KCa2.3 (SK3) in mouse dopaminergic neuronsImmunohistochemical staining of mouse dopaminergic neurons using Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025). A. KCa2.3 is detected in substantia nigra pars compacta. B. Tyrosine hydroxylase staining shows dopaminergic neurons. Triangles point at cells with co-localization.
- CHO-K1 cells expressing human SK3 (1:1000) (Terstappen, G.C. et al. (2001) Neuropharmacology 40, 772.).
- Kohler, M. et al. (1996) Science 273, 1709.
- Xia, X.M. et al. (1998) Nature 395, 503.
- Stocker, M. (2004) Nat. Rev. Neurosci. 5, 758.
KCa2.3 (KCNN3, SK3) is a member of the Ca2+-activated K+ channel family with small conductance that includes KCa2.1 (KCNN1, SK1) and KCa2.2 (KCNN2, SK2). The channel is voltage insensitive and is activated by intracellular Ca2+ in the submicromolar range. It has, though, a similar topology to that of voltage-dependent K+ channels (KV channels), that is six transmembrane domains and intracellular N- and C-termini. The functional channel of all the KCa2 family members is a multimeric protein composed of four pore-forming subunits.
KCa2 channels are extremely sensitive to the levels of intracellular Ca2+ and concentrations as low as 300-700 nM can open the channels very rapidly (5-15 ms). Hence, the KCa2 channels are highly sensitive and fast Ca2+ sensors resembling other known Ca2+-binding proteins. This type of Ca2+-dependent activation is achieved by the constitutive binding of the KCa2 channels to calmodulin, a highly expressed Ca2+-binding protein via a calmodulin-binding domain situated at the cytoplasmic C-terminus.
Pharmacologically, the KCa2 channels are the only known targets of the bee venom toxin Apamin, with KCa2.1 being the least sensitive, KCa2.2 the most sensitive and KCa2.3 showing intermediate sensitivity.
KCa2.3 is predominantly expressed in the nervous system although expression in endothelial cells, heart and liver have been described.
KCa2.3 is known to be involved in the regulation of neuronal excitability. They do so mainly via a phenomenon known as after hyperpolarization in which KCa2 channels open in response to increased intracellular Ca2+ concentrations that result from the entry of extracellular Ca2+ through voltage-dependent Ca2+ channels during action potentials. In this way, KCa2 channels effectively form a Ca2+-mediated feedback loop.
KCa2.3 is involved in the control of firing rate and subsequent dopamine secretion from midbrain dopaminergic neurons. Since malfunction of these neurons is involved in several pathological disorders such as Parkinson’s disease and Schizophrenia, modulators of the KCa2.3 channels have been proposed to be of therapeutic value in these diseases.
Application key:
Species reactivity key:
Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025) is a highly specific antibody directed against an intracellular epitope at the N-terminus of the human KCNN3 channel. The antibody can be used in western blot, immunocytochemistry, and immunohistochemistry applications. It has been designed to recognize KCNN3 from human, rat, and mouse samples.
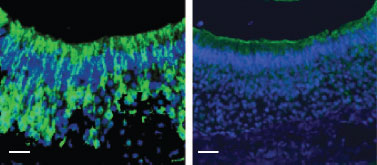 Knockout validation of Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody in mouse VNO neurons.Immunohistochemical staining of in VNO sections from Sk3T/T mice (conditional SK3 knockout mouse strain) fed with (right panel) or without (left panel) DOX diet using Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025).Adapted from Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. with permission of Nature America.
Knockout validation of Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody in mouse VNO neurons.Immunohistochemical staining of in VNO sections from Sk3T/T mice (conditional SK3 knockout mouse strain) fed with (right panel) or without (left panel) DOX diet using Anti-KCNN3 (KCa2.3, SK3) (N-term) Antibody (#APC-025).Adapted from Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. with permission of Nature America.
Applications
Citations
- Mouse brain lysate.
Deng, P.Y. et al. (2019) J. Neurosci. 39, 28. - Mouse CCDcl1 cell lysate (1:1000).
Li, Y. et al. (2016) PLoS ONE 11, e0155006. - Mouse mesenteric arteries lysate.
Yap, F.C. et al. (2016) Am. J. Physiol. 310, H1151. - Rat brain lysate (1:500).
Larson, R.A. et al. (2015) Am. J. Physiol. 308, H1547. - Mouse kidney lysate (1:00).
Berrout, J. et al. (2014) PLoS ONE 9, e95149. - Human iPS cells (1:500).
Linta, L. et al. (2013) Ann. Anat. 195, 303. - Mouse and human uterine tissue (1:100).
Pierce, S.L. and England, S.K. (2010) Am. J. Physiol. 299, E640. - Rat mesenteric arteries.
Hilgers, R.H. et al. (2010) J. Pharmacol. Exp. Ther. 333, 210.
- Rat cortical collecting duct cells.
Pizzoni, A. et al. (2018) J. Cell Biochem. 119, 4120.
- Rat DRG sections (1:1000).
Jager, S.B. et al. (2018) J. Neurosci. Methods 297, 1. - Mouse mesenteric artery sections. Also tested in SK3-/- mice.
Yap, F.C. et al. (2016) Am. J. Physiol. 310, H1151. - Mouse kidney sections (1:100).
Berrout, J. et al. (2014) PLoS ONE 9, e95149. - Rat trigeminal ganglion (1:100).
Donegan, M. et al. (2013) Glia 61, 2000. - Mouse VNO neurons. Also tested in conditional KO mice.
Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. - Rat mesenteric artery paraffin-embedded sections.
Hilgers, R.H. et al. (2010) J. Pharmacol. Exp. Ther. 333, 210.
- Rat dissociated DRGs (1:100).
Jager, S.B. et al. (2018) J. Neurosci. Methods 297, 1. - Human iPS cells (1:100).
Linta, L. et al. (2013) Ann. Anat. 195, 303. - CHO-K1 cells expressing human SK3 (1:1000).
Terstappen, G.C. et al. (2001) Neuropharmacology 40, 772.
- Gui, L. et al. (2013) Am. J. Physiol. 304, H118.
- Bagher, P. et al. (2012) Proc. Natl. Acad. Sci. U.S.A. 109, 18174.
- Sorensen, C.M. et al. (2011) Pflugers Arch. 462, 655.
- Wang, W. et al. (2011) Neuropharmacology 60, 901.
- Hopf, F.W. et al. (2010) Neuron 65, 682.
- Feng, J. et al. (2008) Circulation 118, S46.
- Pierce, S.L. et al (2008) Biol. Reprod. 78, 1058.
- Absi, M. et al. (2007) Br. J. Pharmacol. 151, 332.
- Brown, A. et al. (2007) Am. J. Physiol. 292, C832.
- Liebau, S. et al. (2007) J. Neurochem. 101, 1338.
- McNeish, A.J. et al. (2006) Stroke 37, 1277.
- Moriguchi, S. et al. (2006) Proc. Natl. Acad. Sci. 103, 10811.
- Sandow, S.L. et al. (2006) J. Anat. 209, 689.
- Yamazaki, D. et al. (2006) J. Biol. Chem. 281, 38430.
- Weston, A.H. et al. (2005) Circ. Res. 97, 391.
- Bond, C.T. et al. (2004) J. Neurosci. 24, 5301.
- Kolski Andreaco, A. et al. (2004) J. Biol. Chem. 279, 6893.
- Tamarina, N.A. et al. (2003) Diabetes 52, 2000.
- Taylor, M.S. et al. (2003) Circ. Res. 93, 124.
- Vanderwinden, J.M. et al (2002) Cell Tissue Res. 310, 349.
- Barfod, E.T. et al. (2001) Am. J. Physiol. 280, C836.
- Khanna, R. et al. (2001) Am. J. Physiol. 280, C796.
- Tacconi, S. et al. (2001) Neuroscience 102, 209.
