Overview
- Peptide (C)KQEEKQNRRGLAG, corresponding to amino acid residues 619-631 of rat Slack (Accession Q9Z258). Intracellular, C-terminal.
- Rat and mouse samples (1:200).
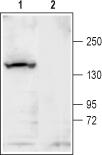 Western blot analysis of rat brain membrane:1. Anti-KCNT1 (Slack) Antibody (#APC-124), (1:200).
Western blot analysis of rat brain membrane:1. Anti-KCNT1 (Slack) Antibody (#APC-124), (1:200).
2. Anti-KCNT1 (Slack) Antibody, preincubated with KCNT1/Slack Blocking Peptide (#BLP-PC124). Western blot analysis of mouse brain membrane:1. Anti-KCNT1 (Slack) Antibody (#APC-124), (1:200).
Western blot analysis of mouse brain membrane:1. Anti-KCNT1 (Slack) Antibody (#APC-124), (1:200).
2. Anti-KCNT1 (Slack) Antibody, preincubated with KCNT1/Slack Blocking Peptide (#BLP-PC124).
KCa4.1 (Na+-activated K+ channel, Slack, Slo2.2, KCNT1) is a member of a subgroup within the Ca2+ activated K+ channel family. The Slack (Sequence like a Ca2+-activated K+ channel) and Slick (Sequence like an intermediate conductance K+ channel, KCNT2) genes, which encode Na+-activated K+ (KNa) channels, are expressed at high levels in neurons in several areas of the nervous system1.
Although KCa4.1 is functionally a Na+-activated K+ channel, it is termed KCa by the IUPHAR nomenclature, due to its sequence homology to other KCa channels.
The family’s protein topology consists of six transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels, the functional unit for KCa4.1 channels is composed of four subunits that can assemble as either homo- or heteromers with KCNT2 (Slick). KCa4.1 and Slick subunits coassemble to form heteromeric channels that differ from the homomers in their unitary conductance, kinetic behavior, subcellular localization, and response to activation of protein kinase C. Heteromer formation requires the N-terminal domain of Slack-B, one of the alternative splice variants of the KCa4.1 channel. This cytoplasmic N-terminal domain of Slack-B also facilitates the localization of heteromeric KNa channels to the plasma membrane2.
K+ channels sensitive to intracellular Na+, termed KNa channels, shape neuronal excitability. KNa channels contribute to the adaptation of firing rates and to slow after-hyperpolarizations that follow repetitive firing. In certain neurons, they also appear to be activated by Na+ influx accompanying single action potentials. Their molecular properties also suggest that these channels contribute to the response of neurons to hypoxia1. KCa4.1 channels were shown to be expressed also in dorsal root ganglion (DRG) neurons, where these channels were found to contain a NAD+ binding sequence and to be modulated by this endogenous compound3.
