Overview
- Peptide (C)IVEDWQQKYRSQILHLEDSK, corresponding to amino acid residues 88-107 of rat KCNE2 (Accession P63161). Intracellular, C-terminal domain.
- Rat heart membranes (1:200). Human ventricle lysate (Zhang, M. et al. (2012) Am. J. Physiol. 302, H910.).
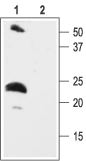 Western blot analysis of rat heart membranes:1. Anti-KCNE2 (MiRP1) Antibody (#APC-054), (1:200).
Western blot analysis of rat heart membranes:1. Anti-KCNE2 (MiRP1) Antibody (#APC-054), (1:200).
2. Anti-KCNE2 (MiRP1) Antibody, preincubated with KCNE2/MiRP1 Blocking Peptide (#BLP-PC054).
- Rat sinoatrial cells (SANs) (Qu, J. et al. (2004) J. Biol. Chem. 279, 43497.).
- Mouse heart.
- Rabbit carotid body chemoreceptor cells (Colinas, O. et al. (2008) J. Gen. Physiol. 131, 455.).
KCNE2 (or MiRP1) is a member of a family of proteins that regulate the activity of voltage-dependent K+ channels. Other members of the family are KCNE1 (IsK, MiNK), KCNE3 (MiRP2), KCNE4 (MiRP3) and KCNE5 (MiRP4).
KCNE1 is the founding member of the family and KCNE2 was discovered based on its homology with KCNE1.1,2
The KCNE regulatory subunits are small proteins (14-20 kD) with a type-1 integral membrane topology. It is believed that both the cytoplasmic C-terminus tail and the transmembrane domain are necessary for the interaction with the a subunits. The stoichiometry of the KCNE subunits with their partner a subunits in the native channels is not clear and ratios ranging from 2 to 14 KCNE subunits per 4 a subunits have been proposed.1,2
KCNE2 is broadly distributed with prominent expression in the brain and heart, but also in other tissues such as skeletal muscle, pancreas and kidney.
The best described functional association of KCNE2 is with the voltage-gated K+ channel KV11.1 (HERG). In the heart, complexes of the two proteins constitute the molecular underlay of the cardiac IKr current and mutations in the KCNE2 subunit are associated with cardiac arrhythmias.1 KCNE2 can form complexes with other channels including the K+ channels KV7.1 (KCNQ1) and KV4.3 and the hyperpolarization-activated cation channels HCN1 and HCN2. In the stomach KV7.1/KCNE2 complexes were found to be crucial for acid secretion as well as for the maintenance of structural integrity, and for parietal cell survival.3
