Overview
- Peptide (C)GYMKSKRREKKSS, corresponding to amino acid residues 56-68 of mouse KCNE4 (Accession Q9WTW3). Intracellular, C-terminus.
- Rat pancreas and heart membrane lysates (1:600- 1:1500).
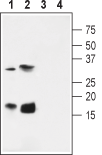 Western blot analysis of rat pancreas membrane (lanes 1 and 3) and heart (lanes 2 and 4) lysates:1,2. Anti-KCNE4 (MiRP3) Antibody (#APC-129), (1:600).
Western blot analysis of rat pancreas membrane (lanes 1 and 3) and heart (lanes 2 and 4) lysates:1,2. Anti-KCNE4 (MiRP3) Antibody (#APC-129), (1:600).
3,4. Anti-KCNE4 (MiRP3) Antibody, preincubated with KCNE4/MiRP3 Blocking Peptide (#BLP-PC129).
The K+ voltage-gated channel subfamily E member (KCNE) family is a group of small, non-conducting, single transmembrane domain proteins that associate with pore-forming potassium channel subunits to form mixed complexes with unique characteristics1.
Five different KCNE proteins have been described (KCNE1-5)2. The KCNE regulatory subunits are small proteins (14-20 kD) with a type-1 integral membrane topology. It is believed that both the cytoplasmic C-terminus tail and the transmembrane domain are necessary for the interaction with the α subunits1.
MinK-related peptides (MiRPs) MiRP3, protein encoded by KCNE43. The importance of these proteins to normal physiology of the heart and nervous system is exposed by their association with clinical disorders such as congenital long QT syndrome, drug-induced cardiac arrhythmias, sensorineural deafness, and periodic paralysis4. In addition, MiRPs influence the normal physiology of endocrine and exocrine glands, intestinal secretion, and renal excretion. MiRP3 is found to co-localize with KV4.2 subunits that contribute to cardiac transient outward K+ currents5. Levels of the KCNE4 transcript in human cardiac ventricle are robust and increase in patients with congestive failure suggesting a regulatory function for MiRP3 in the heart6. In addition to regulating KV4.2, MiRP3 also regulates KCNQ1 and BK channels2,5.
