Overview
- GST fusion protein with the sequence HNFGKTVEVETPHCAMCLYNEKDARARMKRGYDNPNFVLSEVDET DDTQM, corresponding to amino acids 342-391 of rat KCNJ1 (Accession P35560). Intracellular, C-terminus.
 Western blot analysis of rat kidney membranes:1. Anti-KCNJ1 (Kir1.1) Antibody (#APC-001), (1:200).
Western blot analysis of rat kidney membranes:1. Anti-KCNJ1 (Kir1.1) Antibody (#APC-001), (1:200).
2. Anti-KCNJ1 (Kir1.1) Antibody, preincubated with KCNJ1/Kir1.1 Blocking Peptide (#BLP-PC001).- Human submandibular gland (HSG) cells (1:200) (Liu, X. et al. (1999) J. Biol. Chem. 274, 25121.).
- Rat kidney lysate (4 µg Ab/mg protein) (Chen, P. et al. (2006) Am. J. Physiol. 290, C1355.).
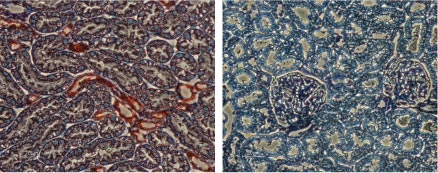 Expression of KCNJ1 in rat kidneyImmunohistochemical staining of rat kidney sections using Anti-KCNJ1 (Kir1.1) Antibody (#APC-001), (left). There is strong staining (red) of tubular epithelial cells in distal tubes. Note that no staining is observed in proximal tubules (arrow). Counterstain of cell nuclei appears blue. A negative control is shown (right).
Expression of KCNJ1 in rat kidneyImmunohistochemical staining of rat kidney sections using Anti-KCNJ1 (Kir1.1) Antibody (#APC-001), (left). There is strong staining (red) of tubular epithelial cells in distal tubes. Note that no staining is observed in proximal tubules (arrow). Counterstain of cell nuclei appears blue. A negative control is shown (right).
- Rat mTAL cells (1:200) (Eng, B. et al. (2007) Am. J. Physiol. 293, F1413.).
- Ho, K. et al. (1993) Nature 362, 31.
- Simon, D.B. et al. (1996) Nat. Genet. 14, 152.
- Wang, W.H. (2006) Am. J. Physiol. 290, F14.
- Lu, Z. and McKinnon, R. (1997) Biochemistry 36, 6936.
Kir1.1 (KCNJ1, ROMK1) was the first member of the family of inward rectifying K+ channels to be cloned.1 The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels, the functional unit for the Kir channel is composed of four subunits that can assemble as either homo or heterotetramers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.3
As its original name indicates (ROMK1, Renal Outer Medullary K+ channel), Kir1.1 is strongly expressed in the kidney in the apical membrane of several kidney segments such as the thick ascending loop of Henle (TAL) and the cortical collecting duct (CCD). In addition, the channel is also expressed in the brain, mainly in the cortex and hippocampus.3
Kir1.1 plays a key role in K+ recycling in the loop of Henle. Indeed, loss-of-function mutations in the Kir1.1 gene cause Bartter’s syndrome type II, a recessive autosomal disease characterized by the impairment of K+ efflux and the subsequent inability of the NKCC2 transporter to continue NaCl uptake. This leads to a high salt concentration in the urine that induces osmotic diuresis and low plasma volume.2
Pharmacologically, the Kir1.1 channel can be inhibited by several general K+ channel blockers such as Tertiapin (#STT-250), however the scorpion toxin Lq2 (#RTL-550) specifically and potently inhibits Kir1.1 channels.4
Application key:
Species reactivity key:
Anti-KCNJ1 (Kir1.1) Antibody (#APC-001) is a highly specific antibody directed against an epitope of the rat potassium channel ROM-K. The antibody can be used in western blot, immunohistochemistry, immunocytochemistry, and immunoprecipitation applications. It has been designed to recognize ROMK1 from rat, mouse, and human samples.
Applications
Citations
- Western blot analysis of mouse kidney lysate. Tested in Kir1.1-/- mice.
Liu, B.C. et al. (2015) J. Am. Nephrol. 26, 1576.
- Mouse kidney lysate.
Yoshioka, W. et al. (2016) Am. J. Physiol. 311, F752. - Mouse kidney lysate.
Liu, B.C. et al. (2015) J. Am. Soc. Nephrol. 26, 1576. - Mouse kidney lysate (1:800).
Todkar, A. et al. (2015) J. Am. Soc. Nephrol. 26, 425. - Rat cortex and medulla lysates (1:2000).
Rengarajan, S. et al. (2014) Am. J. Physiol. 306, F1059. - Mouse kidney cortex.
Vitzthum, H. et al. (2014) J. Physiol. 592, 1139. - Human submandibular gland (HSG) cells (1:200).
Liu, X. et al. (1999) J. Biol. Chem. 274, 25121.
- Rat kidney lysate (4 µg Ab/mg protein).
Chen, P. et al. (2006) Am. J. Physiol. 290, C1355.
- Mouse kidney sections (1:4000).
Todkar, A. et al. (2015) J. Am. Soc. Nephrol. 26, 425.
- Hsieh, S.C. et al. (2008) Rheumatology 47, 150.
- Huang, C. et al. (2007) Am. J. Physiol. 292, F1073.
- Rieg, T. et al. (2007) Kidney Int. 72, 566.
- Yang, S.S. et al. (2007) Cell Metabol. 5, 331.
- Zhang, X. et al. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9517.
- Bundis, F. et al. (2006) Cell. Physiol. Biochem. 17, 1.
- Stubbe, J. et al. (2006) Am. J. Physiol. 291, F812.
- Zhou, Y. et al. (2006) J. Pharmacol. Exp. Ther. 317, 11.
- Babilonia, E. et al. (2005) J. Biol. Chem. 280, 10790.
- Lin, D.H. et al. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4306.
- O’Connell, A.D. et al. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 9954.
- Lin, D.H. et al. (2004) Am. J. Physiol. 286, F881.
- Sterling, H. et al. (2002) J. Biol. Chem. 277, 4317.
- Michelakis, E.D. et al. (2001) Am. J. Physiol. 280, 1138.
- Wei, Y. el al. (2001) Am. J. Physiol. 281, F206.
- Mennitt, P.A. et al. (2000) Am. J. Physiol. 278, F916.
