Overview
- Peptide (C)EFGS HLDLE RMQAA TLPLD N, corresponding to amino acid residues 418-437 of rat Kir2.3 (Accession P52190). Intracellular, C-terminal part.
- Rat brain membranes (1:200).
Human heart lysate (1:200) (Voigt, N. et al. (2010) Circ. Arrhyth. Electrophysiol. 3, 472.).  Western blot analysis of rat kidney (lanes 1 and 3) and brain (lanes 2 and 4) membranes:1,2. Anti-Kir2.3 (KCNJ4) Antibody (#APC-032), (1:200)
Western blot analysis of rat kidney (lanes 1 and 3) and brain (lanes 2 and 4) membranes:1,2. Anti-Kir2.3 (KCNJ4) Antibody (#APC-032), (1:200)
3,4. Anti-Kir2.3 (KCNJ4) Antibody, preincubated with Kir2.3/KCNJ4 Blocking Peptide (#BLP-PC032).
- Rat brain sections.
- Canine ventricle and atrium myocytes (1:300) (Melnyk, P. et al. (2002) Am. J. Physiol. 283, H1123.).
Kir2.3 is a member of the family of inward rectifying K+ channels. The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels the functional unit for the Kir channels is composed of four subunit that can assembly as either homo or heterotetramers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.
Kir2.3 is a member of the Kir2.x subfamily that includes four members (Kir2.1- Kir2.4) that are characterized by strong inward rectification and high constitutive activity.
Kir2.3 is expressed in a variety of tissues including heart and brain. 2
In the heart, Kir2.3 forms heteromers with Kir2.1 and underlay the IK1 current (at least in some species) that is responsible for setting the resting membrane potential, preventing membrane hyperpolarization due to Na+ pump activity, influencing propagation velocity, altering the electrical space constant, and promoting late phase repolarization.1,2
Application key:
Species reactivity key:
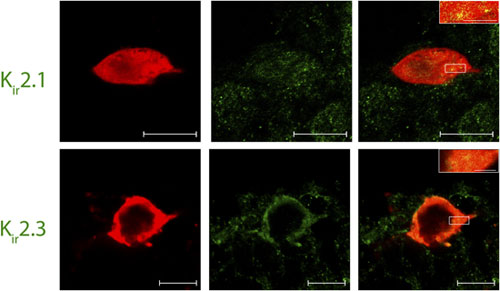 Expression of Kir2.1 and Kir2.3 channels in Lamina I pacemaker cells.Immunohistochemical staining of rat spinal cord sections using Lamina I pacemaker using Anti-Kir2.1 (KCNJ2) Antibody (#APC-026) and Anti-Kir2.3 (KCNJ4) Antibody (#APC-032). Confocal images of representative biocytin-filled pacemaker neurons (red) processed for Kir (green). Merged images demonstrate that immunoreactive puncta for Kir2.1 and Kir2.3 (yellow; inset) are localized to identified pacemaker neurons within lamina I of the neonatal spinal cord (right). Scale bars: 10 μm; inset, 2 μm.Adapted from Li, J. et al. (2013) with permission of The Society for Neuroscience.
Expression of Kir2.1 and Kir2.3 channels in Lamina I pacemaker cells.Immunohistochemical staining of rat spinal cord sections using Lamina I pacemaker using Anti-Kir2.1 (KCNJ2) Antibody (#APC-026) and Anti-Kir2.3 (KCNJ4) Antibody (#APC-032). Confocal images of representative biocytin-filled pacemaker neurons (red) processed for Kir (green). Merged images demonstrate that immunoreactive puncta for Kir2.1 and Kir2.3 (yellow; inset) are localized to identified pacemaker neurons within lamina I of the neonatal spinal cord (right). Scale bars: 10 μm; inset, 2 μm.Adapted from Li, J. et al. (2013) with permission of The Society for Neuroscience.