Overview
- GST fusion protein with the sequence LQRISSVPGNSEEKLVSKTTKMLSDPMSQSVADLPPKLQKMAGGPTRMEGNLPAKLRKMNSDRFT, corresponding to residues 437-501 of mouse GIRK1 (Accession P63250). Intracellular, C-terminus.

- Mouse atrial myocytes (Cui, S. et al. (2010) J. Biol. Chem. 285, 41732.).
- Rat brain sections.
Human skin sections (1:200) (Nockemann, D. et al. (2013) EMBO Mol. Med. 5, 1263.).
- Mouse INS-IE cells (Iwanir, S. and Reuveny, E. (2008) Pflughers Arch. 456, 1097.).
- Dascal, N. (1997) Cell Signal 9, 551.
- Wickman, K. et al. (1998) Neuron 20, 103.
- Mark, M.D. et al. (2000) Eur. J. Biochem. 267, 5830.
- Jin, W. and Lu, Z. (1998) Biochemistry 37, 13291.
- Kubo, Y. et al. (2005) Pharmacol. Rev. 57, 509.
Kir3.1 (or G-protein regulated inward-rectifier K+ channel 1, GIRK1) is a member of the family of inward rectifying K+ channels. The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels, the functional unit for the Kir channels is composed of four subunits that can assemble as either homo- or heterotetramers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.
Kir3.1 is a member of the Kir3.x subfamily that includes four members (Kir3.1- Kir3.4). The Kir3 family is characterized by the fact that the channels can be activated by neurotransmitters and other factors acting via the activation of G-protein coupled receptors. Binding of the corresponding ligand to the G-protein receptor induces the dissociation of Gα-GTP from the Gβγ dimer. The latter directly binds to Kir3 and activates the channel1,3.
In the heart, Kir3.1 co-assembles with Kir3.4 to form the prototypical muscarinic-gated K+ channel KAch current, responsible for slowing the heart rate in response of parasympathetic stimulation2.
In the brain, Kir3.1 co-assembles with Kir3.2 and mediates the inhibitory effects of many neurotransmitters including opioid, adrenergic, muscarinic, dopaminergic and γ-aminobutyric acid (GABA)1,3.
A peptide toxin originating from the Apis mellifera bee venom, Tertiapin (#STT-250) was shown to be a potent blocker of Kir3.1 containing channels (8.6 nM for the Kir3.1/3.4 combination and 5.4 nM for the Kir3.1/3.2)4,5.
Application key:
Species reactivity key:
Anti-GIRK1 (Kir3.1) Antibody (#APC-005) is a highly specific antibody directed against an epitope of the mouse protein. The antibody can be used in western blot, immunprecipitation, immunocytochemistry, and immunohistochemistry applications. It has been designed to recognize Kir3.1 from human, rat, and mouse samples.
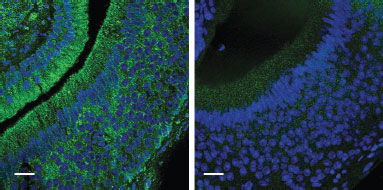
Knockout validation of Anti-GIRK1 (Kir3.1) Antibody in mouse VNO neuronsImmunohistochemical staining of wild-type (left panel) and Girk1–/– (right panel) VNO neurons using Anti-GIRK1 (Kir3.1) Antibody (#APC-005).Adapted from Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. with permission of Nature America.
Applications
Citations
- Immunohistochemical staining of mouse VNO neurons. Also tested in KO mice.
Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. - Western blot analysis of mouse brain lysates. Also tested in GIRK1-/- mice.
Koyrakh, L. et al. (2005) J. Neurosci. 25, 11468.
- Human heart and cardiomyocyte lysates.
Morishima, M. et al. (2016) Circ. J. 80, 1346. - Mouse INS-1 cells lysates.
Wu, Y. et al. (2015) J. Biol. Chem. 290, 29676. - Mouse brain lysate (1:1000).
Goodfellow, N.M. et al. (2014) J. Neurosci. 34, 6107. - Rat cardiomyocytes.
Bingen, B.O. et al. (2013) Circulation 128, 2732. - Mouse brain lysates. Also tested in GIRK1-/- mice.
Koyrakh, L. et al. (2005) J. Neurosci. 25, 11468.
- Mouse atrial myocytes.
Cui, S. et al.(2010) J. Biol. Chem. 285, 41732.
- Rat brain sections.
Dell’Orco, J.M. et al. (2015) J. Neurosci. 35, 11292. - Mouse and Rat DRG, sciatic nerve and skin sections (1:200).
Nockemann, D. et al. (2013) EMBO Mol. Med. 5, 1263. - Human skin sections (1:200).
Nockemann, D. et al. (2013) EMBO Mol. Med. 5, 1263. - Rat heart sections (1:20).
Atkinson, A.J. et al. (2013) J. Am. Heart Assoc. 2, e000246. - Mouse VNO neurons. Also tested in KO mice.
Kim, S. et al. (2012) Nat. Neurosci. 15, 1236.
- Mouse INS-IE cells.
Iwanir, S. and Reuveny, E. (2008) Pflughers Arch. 456,1097.
- Booker, S.A. et al. (2016) Cereb. Cortex 27, 2318.
- Robertson, D.N. et al. (2016) Methods 92, 19.
- Liang, B. et al. (2014) Cardiovasc. Res. 101, 175.
- Christopherson, I.E. et al. (2013) Eur. Heart J. 34, 1517.
- Marker, C.L. et al. (2006) J. Neurosci. 26, 12251.
- Marker, C.L. et al. (2005) J. Neurosci. 25, 3551.
- Rishal, I. et al. (2005) J. Biol. Chem. 280, 16685.
- Sarac, R. et al. (2005) J. Neurosci. 25, 1836.
- Ehrlich, J.R. et al. (2004) J. Physiol. 557, 583.
- Shankar, H. et al. (2004) Blood 104, 1335.
- Derjean, D. et al. (2003) Nat. Neurosci. 6, 274.
- Dobrzynski, H. et al. (2002) Am J. Physiol. 283, H615.
- Lavine, N. et al. (2002) J. Biol. Chem. 277, 46010.
- Peleg, S. et al. (2002) Neuron 33, 87.
- Fili, O. et al. (2001) J. Neurosci. 21, 1964.
- Jelacic, T.M. et al. (2000) J. Biol. Chem. 275, 36211.
- Lei, Q. et al. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 9771.
- Singer-Lahat, D. et al. (2000) Pflugers Arch. 440, 627.
- Pei, Q. et al. (1999) Neuroscience 90, 621.
- Kennedy, M.E. et al. (1999) J. Biol. Chem. 274, 2571.
- Kuzhikandathil, E.V. et al. (1998) Mol. Cell. Neurosci. 12, 390.

