Overview
- GST fusion protein with the sequence LQRISSVPGNSEEKLVSKTTKMLSDPMSQSVADLPPKLQKMAGGPTRMEGNLPAKLRKMNSDRFT, corresponding to residues 437-501 of mouse GIRK1 (Accession P63250). Intracellular, C-terminus.
- Mouse atrial myocytes (Cui, S. et al. (2010) J. Biol. Chem. 285, 41732.).
- Rat brain sections.
Human skin sections (1:200) (Nockemann, D. et al. (2013) EMBO Mol. Med. 5, 1263.).
- Mouse INS-IE cells (Iwanir, S. and Reuveny, E. (2008) Pflughers Arch. 456, 1097.).
Kir3.1 (or G-protein regulated inward-rectifier K+ channel 1, GIRK1) is a member of the family of inward rectifying K+ channels. The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels, the functional unit for the Kir channels is composed of four subunits that can assemble as either homo- or heterotetramers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.
Kir3.1 is a member of the Kir3.x subfamily that includes four members (Kir3.1- Kir3.4). The Kir3 family is characterized by the fact that the channels can be activated by neurotransmitters and other factors acting via the activation of G-protein coupled receptors. Binding of the corresponding ligand to the G-protein receptor induces the dissociation of Gα-GTP from the Gβγ dimer. The latter directly binds to Kir3 and activates the channel1,3.
In the heart, Kir3.1 co-assembles with Kir3.4 to form the prototypical muscarinic-gated K+ channel KAch current, responsible for slowing the heart rate in response of parasympathetic stimulation2.
In the brain, Kir3.1 co-assembles with Kir3.2 and mediates the inhibitory effects of many neurotransmitters including opioid, adrenergic, muscarinic, dopaminergic and γ-aminobutyric acid (GABA)1,3.
A peptide toxin originating from the Apis mellifera bee venom, Tertiapin (#STT-250) was shown to be a potent blocker of Kir3.1 containing channels (8.6 nM for the Kir3.1/3.4 combination and 5.4 nM for the Kir3.1/3.2)4,5.
Application key:
Species reactivity key:
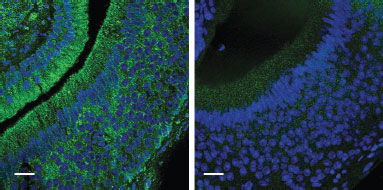
Knockout validation of Anti-GIRK1 (Kir3.1) Antibody in mouse VNO neuronsImmunohistochemical staining of wild-type (left panel) and Girk1–/– (right panel) VNO neurons using Anti-GIRK1 (Kir3.1) Antibody (#APC-005).Adapted from Kim, S. et al. (2012) Nat. Neurosci. 15, 1236. with permission of Nature America.

