Overview
- GST fusion protein with the sequence ELANRAEVPLSWSVS SKLNQHAELETEEEEKNPEELTERNG, corresponding to residues 374-414 of mouse Kir3.2 (Accession P48542). Intracellular, C-terminal part.
- Mouse brain membrane proteins (1:174) (Jelacic, T.M. et al. (2000) J. Biol. Chem. 275, 36211.).
- Rat brain and mouse brain sections (1:400).
Mouse spinal cords (Marker, C.L. et al. (2004) J. Neurosci. 24, 2806.).
- hVM1 (human ventral mesencephalic neural stem cell line 1 (1:400) (Courtois, E.T. et al. (2010) J. Biol. Chem. 285, 9881.).
Kir3.2 (or G-protein regulated inward-rectifier K+ channel, GIRK2) is a member of the family of inward rectifying K+ channels. The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels, the functional unit for the Kir channels is composed of four subunits that can assemble as either homo- or heterotetramers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.
Kir3.2 is a member of the Kir3.x subfamily that includes four members (Kir3.1- Kir3.4). The Kir3 family is characterized by the fact that the channels can be activated by neurotransmitters and other factors acting via the activation of G-protein coupled receptors. Binding of the corresponding ligand to the G-protein receptor induces the dissociation of Gα-GTP from the Gbg dimer. The latter directly binds to Kir3 and activates the channel.2,3
Kir3.2 is mainly expressed in the brain, where it co-assembles with Kir3.1 (GIRK1) or Kir3.3 (GIRK3) and mediates the inhibitory effects of many neurotransmitters including opioid, adrenergic, muscarinic, dopaminergic and GABAergic neurotransmitters.2,3
Point mutations in the mouse Kir3.2 channel cause the weaver (wv) phenotype, a neurological abnormality characterized by the abnormal ‘weaving’ of the mice when they walk, hence the name weaver which is due to a substantial loss of cerebellar granule neurons. These mice also display mild local motor hyperactivity, presumably caused by the degeneration of dopaminergic neurons in the substantia nigra, spontaneous seizures and male sterility.1
A peptide toxin originating from the Apis mellifera bee venom, Tertiapin (#STT-250) was shown to be a potent blocker of Kir3.2 containing channels (7 nM for Kir3.2 alone and 5.4 nM for the Kir3.1/3.2 combination).4
Application key:
Species reactivity key:
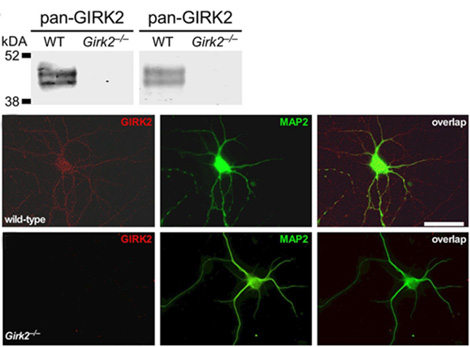
Knockout validation of Anti-GIRK2 (Kir3.2) Antibody in mouse hippocampus.Western blot analysis of mouse hippocampus lysate using Anti-GIRK2 (Kir3.2) Antibody (#APC-006) (upper panels). Note lack of GIRK2 in knockout mice. Immunocytochemical staining of cultured hippocampal mouse neurons with Anti-GIRK2 (Kir3.2) Antibody in wild type cells (upper staining) and Girk2-/- cells (lower staining).Adapted from Marron Fernandez de Velasco, E. et al. (2017) Sci. Rep. 7, 1639. with permission of SPRINGER NATURE.

