Overview
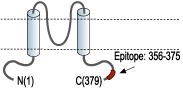
- Peptide (C)KLEE SLREQ AEKEG SALSV R, corresponding to amino acid residues 356-375 of rat Kir4.1 (Accession P49655). Intracellular, C-terminus.
 Western blot analysis of rat brain membranes:1. Anti-Kir4.1 (KCNJ10) Antibody (#APC-035), (1:400).
Western blot analysis of rat brain membranes:1. Anti-Kir4.1 (KCNJ10) Antibody (#APC-035), (1:400).
2. Anti-Kir4.1 (KCNJ10) Antibody, preincubated with Kir4.1/KCNJ10 Blocking Peptide (#BLP-PC035).
- Rat renal cortex lysate (Cha, S.K. et al. (2011) J. Biol. Chem. 286, 1828.).
- Rat brain sections.
Mouse hippocampus (1:200) (Hsu, M.S. et al. (2011) Neuroscience 178, 21.).
Human brain tissue (Saadoun, S. et al. (2003) J. Clin. Pathol. 56, 972.).
- Mouse outer hair cells (OHCs) (Ruttiger, L. et al. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12922.).
- Takumi, T. et al. (1995) J. Biol. Chem. 270, 16339.
- Higashi, K. et al. (2001) Am. J. Physiol. 281, C922.
- Butt, A.M. and Kalsi, A. (2006) J. Cell Mol. Med. 10, 33.
Kir4.1 is a member of the inward rectifying K+ channel family. The family includes 15 members that are structurally and functionally different from the voltage-dependent K+ channels.
The family’s topology consists of two transmembrane domains that flank a single and highly conserved pore region with intracellular N- and C-termini. As is the case for the voltage-dependent K+ channels the functional unit for the Kir channels is composed of four subunit that can assembly as either homo or heteromers.
Kir channels are characterized by a K+ efflux that is limited by depolarizing membrane potentials thus making them essential for controlling resting membrane potential and K+ homeostasis.
Kir4.1 is a member of the Kir4 subfamily that includes one other member: Kir4.2. Kir4.1 can co-assemble with Kir4.2 but also with other Kir channels such as Kir2.1 and Kir5.1.
The Kir4 subfamily has been classified as weak rectifiers with intermediate conductance.
Kir4.1, encoded by KCNJ10, is mainly expressed in brain, specifically in glia cells, but also in retina, ear and kidney.1,2
It has been proposed that Kir4.1 has an essential role in glial K+ buffering, a process that re-uptakes the K+ released during neuronal activity into the intracellular interstitial space. Loss of Kir4.1 causes retinal defects and loss of endochoclear potential.3
Application key:
Species reactivity key:
Anti-Kir4.1 (KCNJ10) Antibody (#APC-035) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize Kir4.1 potassium channel from rat, mouse, and human samples.
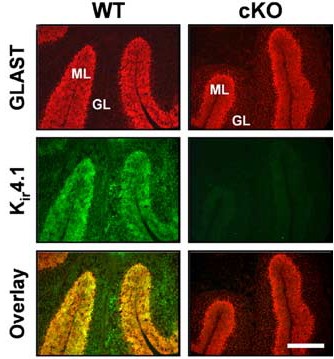
Knockout validation of Anti-Kir4.1 (KCNJ10) Antibody in mouse cerebellum.Immunohistochemical staining of mouse cerebellum sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). Kir4.1 staining (green). GLAST staining (red) co-localizes with Kir4.1 (overlay panel). Note the lack of Kir4.1 staining in Kir4.1 conditional knockout cerebellum.Adapted from Djukic, B. et al. (2007) J. Neurosci. 27, 11354. with permission of the Society for Neuroscience.
Applications
Citations
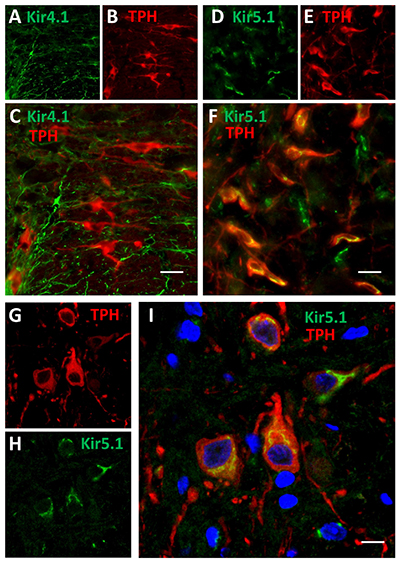 Expression of Kir4.1 and Kir5.1 channels in rat brainstem.Immunohistochemical staining of rat brainstem sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035) and Anti-Kir5.1 Antibody (#APC-123) showed that both channels are expressed (green). Kir4.1 staining is limited to astrocytes, while that of Kir5.1 is detected in 5-HT neurons together with the 5-HT neuronal marker TPH (red).
Expression of Kir4.1 and Kir5.1 channels in rat brainstem.Immunohistochemical staining of rat brainstem sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035) and Anti-Kir5.1 Antibody (#APC-123) showed that both channels are expressed (green). Kir4.1 staining is limited to astrocytes, while that of Kir5.1 is detected in 5-HT neurons together with the 5-HT neuronal marker TPH (red).
Adapted from Puissant, M.M. et al. (2017) Front. Cell. Neurosci. 11, 34. with permission of Frontiers.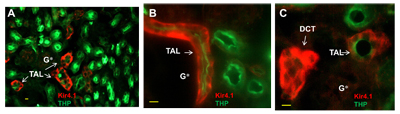 Expression of Kir4.1 in mouse kidney sections.Immunohistochemical staining of mouse kidney sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). Kir4.1 expression (red) is detected in the basolateral membrane of the cortical thick ascending limb.
Expression of Kir4.1 in mouse kidney sections.Immunohistochemical staining of mouse kidney sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). Kir4.1 expression (red) is detected in the basolateral membrane of the cortical thick ascending limb.
Adapted from Zhang, C. et al. (2015) with permission of the American Physiologycal Society.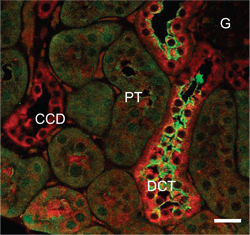 Expression of Kir4.1 in mouse kidney.Immunohistochemical staining of mouse kidney sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). Kir4.1 staining (red) is localized to the distal convoluted tubes and co-localizes with NCC (sodium-chloride co-transporter), (green).
Expression of Kir4.1 in mouse kidney.Immunohistochemical staining of mouse kidney sections using Anti-Kir4.1 (KCNJ10) Antibody (#APC-035). Kir4.1 staining (red) is localized to the distal convoluted tubes and co-localizes with NCC (sodium-chloride co-transporter), (green).
Adapted from Zaika, O. et al. (2016) with permission of the American Physiological Society.
- Immunohistochemical staining of mouse brain sections. Tested in Kir4.1-/- mice.
Brasko, C. et al. (2017) Brain Struct. Funct. 222, 41. - Immunohistochemical staining of mouse oligodendrocyte sections. Tested on Kir4.1-/- cells.
Battefeld, A. et al. (2016) Nat. Commun. 7, 11298. - Immunohistochemical staining of mouse cerebellum sections. Tested in Kir4.1 conditional knockout cerebellum.
Djukic, B. et al. (2007) J. Neurosci. 27, 11354.
- Human primary astrocytes.
Limbad, C. et al. (2020) PloS ONE 15, e0227887. - Rat habenular membrane lysate (1:1000).
Cui, Y. et al. (2018) Nature 554, 323. - Mouse spinal cord lysate.
Kelley, K.W. et al. (2018) Neuron 98, 306. - Rat brain lysate (1:100).
Puissant, M.M. et al. (2017) Front. Cell. Neurosci. 11, 34. - Rat kidney lysate.
Blankenstein, K.I. et al. (2017) Am. J. Physiol. 312, F489. - Rat hippocampus lysate (1:1000).
Puttachary, S. et al. (2016) Neurobiol. Dis. 93, 184. - Mouse cornea lysate.
Zhang, C. et al. (2016) Am. J. Physiol. 310, C993. - Mouse spinal cord lysate (1:750).
Minkel, H.R. et al. (2015) Glia 63, 2285. - Mouse mMCD3 cell lysate (1:500).
Slaats, G.G. et al. (2015) J. Cell. Sci. 128, 4550. - Rat Müller cell lysate (1:200).
Chen, C. et al. (2014) Graefes Arch. Clin. Exp. Ophthalmol. 252, 51. - Mouse brain lysate (1:2000).
Nakajima, M. et al. (2013) Brain Res. 1537, 340. - Mouse cerebellum lysate (1:500).
Winkler, U. et al. (2013) Glia 61, 1067. - Rat brain lysate (1:500).
Harada, Y. et al. (2013) Brain Res. 1517, 141.
- Rat renal cortex lysate.
Cha, S.K. et al. (2011) J. Biol. Chem. 286, 1828.
- Mouse ventral spinal cord sections.
Kelley, K.W. et al. (2018) Neuron 98, 306. - Mouse brain sections. Also tested in Kir4.1-/- mice.
Brasko, C. et al. (2017) Brain Struct. Funct. 222, 41. - Rat brainstem sections (1:1000).
Puissant, M.M. et al. (2017) Front. Cell. Neurosci. 11, 34. - Human temporal bone sections.
Lopez, I.A. et al. (2016) Histochem. Cell Biol. 146, 367. - Rat brain sections (1:250).
Puttachary, S. et al. (2016) Neurobiol. Dis. 93, 184. - Mouse oligodendrocyte sections. Also tested in Kir4.1-/-cells.
Battefeld, A. et al. (2016) Nat. Commun. 7, 11298. - Mouse cochlea sections (1:300).
Shibata, S. et al. (2016) J. Neurosci. 36, 8200. - Rat trigeminal sections (1:4000).
Takeda, M. et al. (2015) Neuroscience 288, 51. - Mouse DRG sections (1:2500).
Rajasekhar, P. et al. (2015) J. Biol. Chem. 290, 29051. - Mouse kidney sections.
Wang, L. et al. (2015) J. Am. Soc. Nephrol. 26, 2678. - Rat kidney sections (1:200).
Slaats, G.G. et al. (2015) J. Cell. Sci. 128, 4550. - Mouse kidney sections.
Zhang, C. et al. (2015) Am. J. Physiol. 308, F1288. - Mouse ear sections (1:400).
Stevens, S.M. et al. (2014) Otolaryngol. Head Neck Surg. 150, 659. - Mouse brain sections (1:200).
Nakajima, M. et al. (2013) Brain Res. 1537, 340. - Mouse inner ear sections.
Kim, H.J. et al. (2013) J. Neurosci. 33, 14601. - Rat cochlea (1:800).
Takiguchi, Y. et al. (2013) Neurosci. Res. 77, 33. - Mouse cochlea (1:300).
Girotto, G. et al. (2013) PLoS ONE 8, e80323. - Rat formalin-fixed-paraffin-embedded brain sections (1:50-1:100).
Harada, Y. et al. (2013) Brain Res. 1517, 141. - Mouse stria vascularis.
Buniello, A. et al. (2013) PLoS ONE 8, e56274. - Mouse hippocampus (1:200).
Hsu, M.S. et al. (2011) Neuroscience 178, 21. - Mouse cerebellum sections. Also tested in Kir4.1 conditional knockout cerebellum.
Djukic, B. et al. (2007) J. Neurosci. 27, 11354. - Human brain tissue.
Saadoun, S. et al. (2003) J. Clin. Pathol. 56, 972.
- Human iPSC-derived astrocytes (1:2000).
Kelley, K.W. et al. (2018) Neuron 98, 306. - Mouse muller cells.
Vacca, O. et al. (2016) Hum. Mol. Genet. 25, 3070. - Mouse mMCD3 cells (1:200).
Slaats, G.G. et al. (2015) J. Cell. Sci. 128, 4550. - Mouse outer hair cells (OHCs).
Ruttiger, L. et al. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12922.
- Mouse ventral spinal cord sections.
Human iPSC-derived astrocytes (1:2000).
Kelley, K.W. et al. (2018) Neuron 98, 306.
- Lorente Canovas, B. et al. (2013) Dis. Model. Mech. 6, 434.
- Lien, C.F. et al. (2012) J. Biol. Chem. 287, 41374.
- de Melo, J. et al. (2012) Proc. Natl. Acad. Sci. U.S.A. 109, 4657.
- Min, K.J. et al. (2012) J. Neuroinflammation 9, 100.
- Tajada, S. et al. (2012) J. Physiol. 590, 6075.
- Xie, B. et al. (2012) Invest Ophthalmol. Vis. Sci. 53, 1023.
- Brignone, M.S. et al. (2011) Hum. Mol. Genet. 20, 90.
- Tham, D. and Moukhles, H. (2011) Am. J. Physiol. 301, F396.
- Wurm, A. et al. (2011) Invest Ophthalmol. Vis. Sci. 52, 3360.
- Zhao, M. et al. (2011) Invest Ophthalmol. Vis. Sci. 52, 6340.
- Zhang. X. et al. (2011) Am. J. Physiol. 301, C729.
- Reichold, M. et al. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 14490.
- Bockenhauer, D. et al. (2009) N. Engl. J. Med. 360, 1960.
- Mustapha, M. et al. (2009) J. Neurosci. 29, 1212.
- Satz, J.S. et al. (2009) J. Neurosci. 29, 13136.
- Seifert, G. et al. (2009) J. Neurosci. 29, 7474.
- Iandiev, I. et al. (2008) Invest Ophthalmol. Vis. Sci. 49, 3559.
- Trowe, M.O. et al. (2008) Development 135, 1725.
- Vit, J.P. et al. (2008) J. Neurosci. 28, 4861.
- Zhang, H. et al. (2008) Mol. Cell. Neurosci. 37, 1.
- Chen, J. and Nathans, J. (2007) Dev. Cell 13, 325.
- Ivens, S. et al. (2007) Brain 130, 535.
- Ruiz-Ederra, J. et al. (2007) J. Biol. Chem. 282, 21866.
- Iandiev, I. et al. (2006) Invest. Ophthalmol. Vis. Sci. 47, 2161.
- Kaiser, M. et al. (2006) J. Neurochem. 99, 900.
- Mao, X. et al. (2006) Eur. J. Neurosci. 23, 2929.
- Neusch, C. et al. (2006) J. Neurophysiol. 95, 1843.
- Pannicke, T. et al. (2006) Diabetes 55, 633.
- Tiwari-Woodroof. S. et al. (2006) Am. J. Physiol. 291, 687.
- Da, T. and Verkman, A.S. (2004) Invest. Ophthalmol. Vis. Sci. 45, 4477.
- Dalloz, C. et al. (2003) Hum. Mol. Genet. 12, 1543.
- Connors, N.C. and Kofuji, P. (2002) J. Neurosci. 22, 4321.
