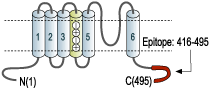
Overview
- GST fusion protein with the sequence HRETEGEEQAQLLHV SSPNLASDSDLSRRSSSTISKSEYMEIEEDMNNSIAHYRQANIRTGNCTTADQNCVNKSKLLTDV, corresponding to amino acid residues 416-495 of mouse KV1.1 (Accession P16388). Intracellular, C-terminus.
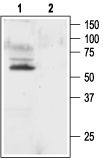 Western blot analysis of rat brain membranes:1. Anti-KV1.1 (KCNA1) Antibody (#APC-009), (1:200).
Western blot analysis of rat brain membranes:1. Anti-KV1.1 (KCNA1) Antibody (#APC-009), (1:200).
2. Anti-KV1.1 (KCNA1) Antibody, preincubated with Kv1.1/KCNA1 Blocking Peptide (#BLP-PC009).
- Rat brain synaptosomes (Fili, O. et al. (2001) J. Neurosci. 21, 1964.).
- Rat brain sections.
Mouse sciatic nerve (1:200) (Zhou, L. et al. (1998) J. Neurosci. 18, 7200.).
- Human neuromas (1:100) (England, J.D. et al. (1998) Neurosci. Lett. 255, 37.).
- Baumann, A. et al. (1988) EMBO J. 7, 2457.
- Gutman, G.A. et al. (2005) Pharmacol. Rev. 57, 473.
- Adelman, J.P. et al. (1995) Neuron 15, 1449.
- Bogin, O. (2006) Modulator 21, 28.
KV1.1 is a mammalian voltage-dependent K+ channel, homologous to the Drosophila Shaker K+ channel. KV1.1 was the first mammalian KV channel to be cloned from mouse brain.1 Eight Shaker-related genes exist in mammals constituting the KV1, subfamily of the large KV channel family of genes.2
A functional KV1 channel is either a membrane spanning homotetramer or heterotetramer, which is composed of members of the same subfamily. In addition several auxiliary subunits and intracellular proteins might interact with the channel and affect its function. The structure of KV1.1 channel is similar to all KV channels and includes six membrane spanning helices creating a voltage sensor domain and a pore domain.2
The channel is expressed in neurons and cardiac and skeletal muscle tissue as well as in the retina and pancreas.2 The functional channel is considered low voltage activated and shows very little inactivation. Therefore, this channel activity influences the membrane potential and excitability of neurons and muscle. Mutations in the coding of KV1.1 gene were discovered in Episodic Ataxia patients.3
KV1.1 channels are sensitive to low doses of TEA (0.3 mM) and 4-AP (0.29 mM), the “classical” non-selective potassium channel blockers.
Several venomous toxins from snakes, scorpions and sea anemones are potent blockers (affecting the channels in the nanomolar range) of KV1.1 channels. Among these, the most potent and selective are α-Dendrotoxin (0.4-4 nM) and δ-Dendrotoxin (0.03-1.8 nM), Dendrotoxin-K (0.03 nM), Agitoxin-2 (0.044 nM) and Hongotoxin-1 (0.031 nM).4
Application key:
Species reactivity key:
Anti-KV1.1 (KCNA1) Antibody (#APC-009) is a highly specific antibody directed against an epitope of the mouse protein. The antibody can be used in western blot, immunohistochemistry, immunocytochemistry, and immunoprecipitation applications. It has been designed to recognize KV1.1 from human, rat, and mouse samples.
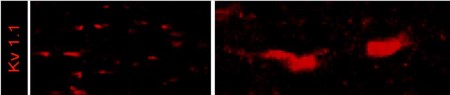
Expression of KV1.1 in mouse optic nerve.Immunohistochemical staining of mouse optic nerve sections using Anti-KV1.1 (KCNA1) Antibody (#APC-009). KV1.1 staining (red) is detected in the juxtaparanode region of the Nodes of Ranvier.Adapted from Bagchi, B. et al. (2014) PLoS ONE 9, e87736. with permission of PLoS.
Applications
Citations
- Human lung carcinoma cell line A549 isolated nuclei.
Jang, S.H. et al. (2015) J. Biol. Chem. 290, 12547.
- Rat brain synaptosomes.
Fili, O., et al. (2001) J. Neurosci. 21, 1964.
- Mouse optic nerve sections.
Bagchi, B. et al. (2014) PLoS ONE 9, e87736. - Mouse sciatic nerve (1:200).
Chen, P. et al. (2014) FASEB J. 28, 1145. - Mouse triangularis sterni muscle sections (1:200).
Yao, D. et al. (2014) J. Neurosci. 34, 880. - Zebrafish embryonic hindbrain (1:200).
Brewster, D.L. and Ali, D.W. (2013) Neurosci. Lett. 539, 54. - Mouse sciatic nerve (1:200).
Zhou, L. et al. (1998) J. Neurosci. 18, 7200.
- Human Neuromas (1:100).
England, J.D. et al. (1998) Neurosci. Lett. 255, 37.
- Ovsepian, S.V. et al. (2013) J. Physiol. 591, 1771.
- Glaudemans, B. et al. (2009) J. Clin. Invest., 119, 936.
- Hsiao, C.F. et al. (2009) J. Neurophysiol. 101, 1407.
- Beraneck, M. et al. (2007) J. Neurosci. 27, 4283.
- Xuan, X. et al. (2007) J. Neurophysiol. 98, 2683.
- Knipper, M. et al. (2006) J. Physiol. 576, 73.
- Leao, R.N. et al. (2006) J. Physiol. 571, 563.
- Sinha, K. et al. (2006) J. Neurophysiol. 95, 1683.
- Devaux, J.J. and Scherer, S.S. (2005) J. Neurosci. 25, 1470.
- Kline, D.D. et al. (2005) J. Neurosci. 25, 3389.
- Wang, J. et al. (2005) Am. J. Physiol. Lung Mol. Cell Physiol. 288, L1049.
- Fukui, I. and Ohmori, H. (2004) J. Neuroscience 24, 7514.
- Karimi-Abdolrezaee, et al. (2004) Eur. J. Neurosci. 19, 577.
- Reid, M.A. et al. (2004) J. Neuroscience 24, 733.
- Dodson, P.D. et al. (2003) J. Physiol. 550.1 27.
- Grunnet, M. et al. (2003) Biophys. J. 85, 1525.
- Koni, P.A. et al. (2003) J. Biol. Chem. 278, 39443.
- Popratiloff, A. et al. (2003) J. Comparative Neurology 461, 466.
- Rios, J.C. et al. (2003) J. Neurosci. 23, 7001.
- Adamson, C.L. et al (2002) J. Neurosci. 22, 1385.
- Altevogt, B.M. et al (2002) J. Neurosci. 22, 6458.
- Arroyo, E.J. et al. (2002) J. Neurosci. 22, 1726.
- Chittajallu, R. et. al. (2002) PNAS 99, 2350.
- Dodson, P.D., et al (2002) J. Neurosci. 22, 6953.
- Felix, R. et al. (2002) Zygote 10, 183.
- Ji, J. et al. (2002) J. Biol. Chem. 277, 20195.
- McCormack, K. et al. (2002) J. Biol. Bhem. 277, 13219.
- Chung, Y.H. et al. (2000) Brain Res.875, 164.
- Keren-Raifman, T. et al. (2000) Biochem Biophys. Res. Commun. 274, 852.
- Nashmi, R. et al. (2000) Eur. J. Neurosci. 12, 491.
- Ouadid-Ahidouch, H., et al. (2000) Biochem. Biophys. Res. Commun. 278, 272.
- Singer-Lahat, D. et al. (2000) Eur J. Physiol. 440, 627.
- Arroyo, E.J. et al. (1999) J. Neurocytol. 28, 333.
- Sobko, A. et al. (1998) J. Neurosci. 18, 10398.
