Overview
- GST fusion protein with the sequence HRETDHEEQAALKEEQGIQRRESGLDTGGQRKVSCSKASFHKTGGPLESTDSIRRGSCPLEKCHLKAKSNVDLRRSLYALCLDTSRETDL, corresponding to amino acid residues 513-602 of mouse KV1.5 (Accession Q61762). Intracellular, C-terminus.
- Perfusion fixed, frozen floating rat and mouse brain sections (1:50).
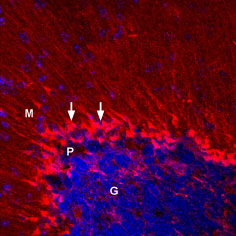 Expression of KV1.5 in mouse cerebellumImmunohistochemical staining of perfusion fixed, free-floating frozen mouse brain sections using Anti-KV1.5 (KCNA5)-ATTO Fluor-550 Antibody (#APC-004-AO), (1:50), (red). Staining was detected in cerebellar Bergmann glial cells (arrows). DAPI is used as the counterstain (blue). G = granule layer, P = Purkinje layer, M = molecular layer.
Expression of KV1.5 in mouse cerebellumImmunohistochemical staining of perfusion fixed, free-floating frozen mouse brain sections using Anti-KV1.5 (KCNA5)-ATTO Fluor-550 Antibody (#APC-004-AO), (1:50), (red). Staining was detected in cerebellar Bergmann glial cells (arrows). DAPI is used as the counterstain (blue). G = granule layer, P = Purkinje layer, M = molecular layer.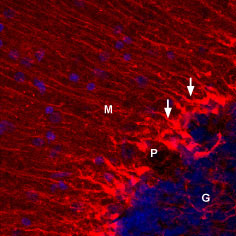 Expression of KV1.5 in rat cerebellumImmunohistochemical staining of perfusion fixed, free-floating frozen rat brain sections using Anti-KV1.5 (KCNA5)-ATTO Fluor-550 Antibody (#APC-004-AO), (1:50), (red). Staining was detected in cerebellar Bergmann glial cells (arrows). DAPI is used as the counterstain (blue). G = granule layer, P = Purkinje layer, M = molecular layer.
Expression of KV1.5 in rat cerebellumImmunohistochemical staining of perfusion fixed, free-floating frozen rat brain sections using Anti-KV1.5 (KCNA5)-ATTO Fluor-550 Antibody (#APC-004-AO), (1:50), (red). Staining was detected in cerebellar Bergmann glial cells (arrows). DAPI is used as the counterstain (blue). G = granule layer, P = Purkinje layer, M = molecular layer.
KV1.5 is a mammalian voltage dependent K+ channel, homologous to the Drosophila Shaker K+ channel. KV1.5 was first cloned from the rat brain.1 Eight Shaker related genes exist in mammals constituting the KV1 subfamily of the large KV channel family of genes.2
A functional KV1 channel is either a membrane spanning homotetramer or heterotetramer, which is composed of members of the same subfamily. In addition several auxiliary subunits and intracellular proteins might interact with the channel and affect its function.
The structure of KV1.5 channel is similar to all KV channels and includes six membrane spanning helices creating a voltage sensor domain and a pore domain.2
The channel is expressed in cardiac and smooth muscle tissue (colon, aorta, stomach and pulmonary artery) as well as in neurons and kidney.2 A loss-of-function mutation in the gene encoding the channel was found in atrial fibrillation patients, stressing its role as a cardiac action potential regulator.3
The functional channel is considered transient (A-type) channel and shows prominent inactivation. Therefore, this channel activity influences the membrane potential and excitability of neurons and muscle.
KV1.5 channels are sensitive to high doses of TEA (330 mM) and low doses of 4-AP (0.27 mM), the “classical” non-selective potassium channel blockers.2
Application key:
Species reactivity key:
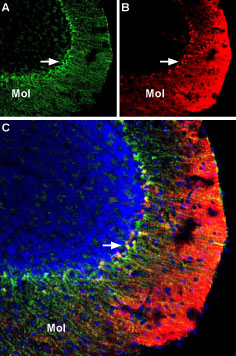
Multiplex staining of KV1.3 and KV1.5 in mouse cerebellumImmunohistochemical staining of mouse perfusion-fixed frozen brain sections using Anti-KV1.3 (KCNA3) (extracellular)-Biotin Antibody (#APC-101-B) (1:400), and Anti-KV1.5 (KCNA5)-ATTO Fluor-550 Antibody (#APC-004-AO), (1:60). A. KV1.3 staining (green) is detected in Bergmann glia soma and processes in the molecular layer (Mol) (arrow). B. Same section shows staining for KV1.5 (red). C. Merge of the two images suggests considerable co-localization in the soma of Bergmann glia. In the molecular layer, the distribution of KV1.5 is diffuse, unlike the discrete staining for KV1.3 in glial processes. Cell nuclei were visualized with DAPI (blue).
