Overview
- Peptide (C)EELPAGAPELPQDGPT, corresponding to residues 1122-1137 of rat KV11.1 (erg1) (Accession O08962). Intracellular, C-terminal part.
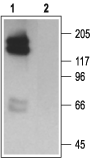 Western blot analysis of HEK 293 cell lysate, stably expressing HERG channels:1. Anti-KCNH2 (erg1) Antibody (1:200).
Western blot analysis of HEK 293 cell lysate, stably expressing HERG channels:1. Anti-KCNH2 (erg1) Antibody (1:200).
2. Anti-KCNH2 (erg1) Antibody, preincubated with KCNH2/erg1 Blocking Peptide (#BLP-PC016).
 Immunoprecipitation of the lysate of HEK 293 cells, stably expressing HERG with Anti-KCNH2 (erg1) Antibody (#APC-016):1. Cell lysate.
Immunoprecipitation of the lysate of HEK 293 cells, stably expressing HERG with Anti-KCNH2 (erg1) Antibody (#APC-016):1. Cell lysate.
2. Cell lysate + protein A beads + Anti-KCNH2 (erg1) Antibody.
3. Cell lysate + protein A beads + pre-immune rabbit serum.
Red arrow indicates the KV11.1 protein while the black arrow shows the IgG heavy chain. Immunoblot was performed with the Anti-KCNH2 (erg1) Antibody.
- Mouse heart sections.
- Mouse cajal cells (Zhu, Y. et al. (2003) Am. J. Physiol. 285, G1249.).
- Curran, M.E. et al. (1995) Cell 80, 795.
- Sanguinetti, M.C. et al. (1995) Cell 81, 299.
- Pardo, L.A. et al. (2004) Physiology 19, 285.
- Gurrola, G.B. et al. (1999) FASEB. J. 13, 953.
- Korolkova, Y.V. et al. (2001) J. Biol. Chem. 276, 9868.
- Zhou, Z. et al. (1998) Biophys. J. 74, 230.
The KV11.1 (HERG) channel is a member of the ether-a-go-go (EAG) subfamily of voltage-dependent K+ channels that includes the related proteins KV11.2 and KV11.3 (erg2 and erg3). KV11.1 possesses the signature structure of the voltage-dependent K+ channels: six membrane-spanning domains and intracellular N- and C-termini.
The KV11.1 current is characterized by strong inward rectification with slow activation and very rapid inactivation kinetics. The channel is expressed in the brain and heart (where it underlies the IKr current) and has a central role in mediating repolarization of action potentials.1,2
Mutations in the KV11.1 channel cause inherited long QT syndrome (LQTS) or abnormalities in the repolarization of the heart that are associated with life-threatening arrhythmias and sudden death. All the identified KV11.1 mutations produce loss of function of the channel via several cellular mechanisms ranging from alterations of gating properties, alterations of channel permeability/selectivity and alterations in intracellular channel trafficking that decreases the number of channels that reach the cell membrane.1,2
Recently, drug-induced forms of LQTS have been reported for a wide range of non-cardiac drugs including antihistamines, psychoactive agents and antimicrobials. All these drugs potently block the KV11.1 channel as an unintended side effect, prompting regulatory drug agencies to issue recommendations for the testing of new drugs for their potential KV11.1 blocking effect.
In addition, KV11.1 expression was found to be upregulated in several tumor cell lines of different histogenesis, suggesting that it confers the cells some advantage in cell proliferation. Indeed, in several studies it has been shown that inhibition of the KV11.1 current leads to a decrease in tumor cell proliferation.3
Several toxins from scorpion venoms are potent blockers (affecting the channels in the nanomolar range) of KV11.1 channels. Among these the most potent and selective are Ergtoxin-1 (16 nM)4 and BeKM-1 (3 nM).5 In addition, the methanesulfonanilide class III antiarrhythmic agent E-4031 also blocks KV11.1 channel in the nanomolar range (7.7 nM).6
Application key:
Species reactivity key:
Anti-KCNH2 (erg1) Antibody (#APC-016) is a highly specific antibody directed against an epitope of the rat KV11.1 channel. The antibody can be used in western blot, immunohistochemistry, immunocytochemistry, and immunoprecipitation applications. It has been designed to recognize KV11.1 from human, rat, and mouse samples.
Applications
Citations
- Immunohistochemistry of mouse inner hair cells. Tested in mice with hair cell-specific deletion of the Kcnh2 gene.
Dierich, M. et al. (2020) Cell Rep. 32, 107869.
- HEK-293 transfected cells.
Zhang, Y. et al. (2016) Biochem. Pharmacol. 113, 24.
- White, E.J. et al. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 295, G691.
- Melnyk, P. et al. (2005) Cardiovasc. Res. 65, 104.
- Bellocq, C. et al. (2004) Mol. Pharmacol. 66, 1093.
- Wang, J. et al. (2004) J. Biol. Chem. 279, 13289.
- Crociani, O. et al. (2003) J. Biol. Chem. 278, 2947.
- Ficker, E. et al. (2003) Circ. Res. 92, 87.
- Milnes, J.T. et al. (2003) Br. J. Pharmacol. 139, 887.
- Papa, M. et al. (2003) J. Comp. Neurol. 466, 119.
- Wang, J. et al. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, 256.
- Zhu, Y. et al. (2003) Am. J. Physiol. 285, G1249.
- Cayabyab, F.S. et al. (2002) J. Biol. Chem. 277, 13673.
- Pond, A.L. et al. (2000) J. Biol. Chem. 275, 5997.
- Akbarali, H.I. et al. (1999) Am. J. Physiol. 277, C1284.
