Overview
- Peptide (C)KRRAIRLANSTAS, corresponding to amino acid residues 538-550 of human KCND1 (Accession Q9NSA2). Intracellular, C-terminal domain.
- Rat and mouse brain lysate and human neurobastoma cell line SH-SY5Y (1:400).
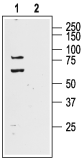 Western blot analysis of rat brain lysate:1. Anti-KV4.1 (KCND1) Antibody (#APC-119), (1:400).
Western blot analysis of rat brain lysate:1. Anti-KV4.1 (KCND1) Antibody (#APC-119), (1:400).
2. Anti- KV4.1 (KCND1) Antibody, preincubated with Kv4.1/KCND1 Blocking Peptide (#BLP-PC119). Western blot analysis of human neuroblastoma cell line SH-SY5Y (lanes 1 and 3) and mouse brain lysate (lanes 2 and 4):1,2. Anti-KV4.1 (KCND1) Antibody (#APC-119), (1:400).
Western blot analysis of human neuroblastoma cell line SH-SY5Y (lanes 1 and 3) and mouse brain lysate (lanes 2 and 4):1,2. Anti-KV4.1 (KCND1) Antibody (#APC-119), (1:400).
3,4. Anti-KV4.1 (KCND1) Antibody, preincubated with Kv4.1/KCND1 Blocking Peptide (#BLP-PC119).
- Human breast cancer tissue (1:200) (Jang, S.H. et al. (2009) Biochem. Biophys. Res. Commun. 384, 180.).
- Mouse dissociated preoptic neurons (Sethi, J. et al. (2011) PLoS ONE 6, e29134.).
KV4.1 is a voltage-dependent K+ channel that belongs to the Shal channel subfamily and includes two other members: KV4.2 and KV4.3.
KV4.1 possesses the signature structure of the voltage-dependent K+ channels: six membrane-spanning domains with intracellular N- and C-termini. As with other members of the voltage-gated K+ channel superfamily, the functional channel is a tetramer that can be composed of more than one member of the Shal subfamily, i.e. heterotetramers of KV4.1 and KV4.2.
The KV4 channels are characterized by activation at subthreshold membrane potentials, inactivate rapidly and recover from inactivation quickly compared with other voltage-dependent K+ channels. This type of current is known as transient A-type K+ currents. For example, depolarization-activated K+ currents in rat neostriatal cholinergic interneurons are predominantly of the A-type and attributable to coexpression of KV4.1 and KV4.2 subunits. The biophysical properties of the KV4.1 subunit can be modified by its association with auxiliary b subunits such as KChIP1 that increase KV4.1 current densities and accelerates both the inactivation and the recovery time.
KV4.1 is highly expressed in the brain but is also expressed in peripheral tissues such as the colon, heart and lung.
The pharmacology of KV4.1 has been less well studied than that of the other KV4 members. KV4.1 is sensitive to the classical K+ channel blockers, 4-aminopyridine and tetraethylammonium. Recently, HmTx1, a peptide blocker derived from the venom of the African tarantula Heteroscodra maculata, has been shown to be a potent KV4.1 inhibitor.
