Overview
- Peptide (C)EFQNEDGEVDDPVLE, corresponding to amino acid residues 209-223 of rat KCNS3 (Accession O88759). 1st extracellular loop.
- Rat lung and mouse heart membranes (1:200-1:1000).
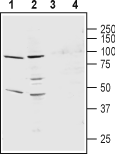 Western blot analysis of rat lung membranes (lanes 1 and 3) and mouse heart membranes (lanes 2 and 4):1,2. Anti-KCNS3 (KV9.3) (extracellular) Antibody (#APC-133), (1:200).
Western blot analysis of rat lung membranes (lanes 1 and 3) and mouse heart membranes (lanes 2 and 4):1,2. Anti-KCNS3 (KV9.3) (extracellular) Antibody (#APC-133), (1:200).
3,4. Anti-KCNS3 (KV9.3) (extracellular) Antibody, preincubated with KCNS3/Kv9.3 (extracellular) Blocking Peptide (#BLP-PC133).
- Rat immersion-fixed, free floating brain frozen sections (1:200).
K+ channels are transmembrane proteins expressed in many excitable and non-excitable cells. Functional entities are formed by the tetrameric assembly of α subunits which could be done in a homomeric or heteromeric fashion. In addition, the association of β subunits is also required for the proper function of K+ channels. Various splice variants are also expressed, thereby complicating the picture1,2.
K+ channels belonging to the KV9 subfamily resemble the delayed-rectifier class of K+ channel α subunits. These channels include six transmembrane domains, an ion selective pore, a leucine zipper and positively charged amino acids in S4 the voltage sensor domain. Interestingly, both KV9.1 and KV9.3 channels are electrically silent delayed rectifying K+ channels. However, they are responsible for modifying the activity of other K+ channels such as that of KV2.1, yielding currents different from those of KV2.1 on its own1.
KV9.1 is mainly expressed in the brain, human lens epithelial cells, kidney, prostate and testis. That of KV9.3 is more generalized and ubiquitous1.
