Overview
- Peptide CEDR(S)EQGTYGND, corresponding to amino acid residues 178 - 190 of mouse MEGF10 (Accession Q6DIB5). Extracellular, N-terminus.
MEGF10 (extracellular) Blocking Peptide (#BLP-NR205)
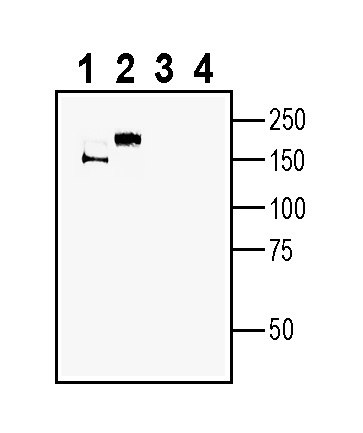 Western blot analysis of mouse brain membranes (lanes 1 and 3) and rat brain membranes (lanes 2 and 4):1-2. Anti-MEGF10 (extracellular) Antibody (#ANR-205), (1:400).
Western blot analysis of mouse brain membranes (lanes 1 and 3) and rat brain membranes (lanes 2 and 4):1-2. Anti-MEGF10 (extracellular) Antibody (#ANR-205), (1:400).
3-4. Anti-MEGF10 (extracellular) Antibody, preincubated with MEGF10 (extracellular) Blocking Peptide (BLP-NR205). Western blot analysis of human U-87 MG glioblastoma cell line lysate (lanes 1 and 4), human MCF-7 breast adenocarcinoma (lanes 2 and 5) and human 1311N1 astrocytoma cell line lysate (lanes 3 and 6):1-3. Anti-MEGF10 (extracellular) Antibody (#ANR-205), (1:400).
Western blot analysis of human U-87 MG glioblastoma cell line lysate (lanes 1 and 4), human MCF-7 breast adenocarcinoma (lanes 2 and 5) and human 1311N1 astrocytoma cell line lysate (lanes 3 and 6):1-3. Anti-MEGF10 (extracellular) Antibody (#ANR-205), (1:400).
4-6. Anti-MEGF10 (extracellular) Antibody, preincubated with MEGF10 (extracellular) Blocking Peptide (BLP-NR205).
MEGF10 is a member of the multiple epidermal growth factor-like domains protein family. MEGF10 is a type I transmembrane protein that consists of 15 EGF-like domains in its extracellular region and is the mammalian homolog of Draper (a Drosophila phagocytosis apoptotic cell receptor)1.
MEGF10 is a mediator for apoptotic cell phagocytosis by central nervous system (CNS) macrophages- microglia and astrocytes, through binding with high affinity to C1q, an eat-me signal, that binds phosphatidylserine expressed on the surface of apoptotic cells2. Also necessary for astrocyte-dependent apoptotic neuron clearance in the developing cerebellum2. In this regard, MEGF10 works alongside other phagocytic receptors expressed in astrocytes or microglia such as the tyrosine kinase receptors MERTK and AXL, the G-protein coupled receptors Bai1 and GPR56 or the TREM2 receptor3.
MEGF10 is involved in the uptake of amyloid-β peptide (Aβ42) in the brain. Alzheimer's disease (AD) is characterized by extracellular accumulation of senile plaques, the major component of which is the amyloid-β peptide (Aβ), and the presence of neurofibrillary tangles. MEGF10 as a functional receptor that mediates the uptake of amyloid-β peptide will help elucidate the molecular mechanisms of amlyoid-β clearance in Alzheimer's disease1,4.
MEGF10 is also an essential factor in the regulation of myogenesis. It controls the balance between skeletal muscle satellite cells proliferation and differentiation through regulation of the notch signaling pathway5. In fact, mutations in the MEGF10 gene are the underlying cause of Early-onset myopathy, areflexia, respiratory distress, and dysphagia (EMARDD), a lethal congenital myopathy characterized by hypotonia with respiratory distress 5,6.
