Overview
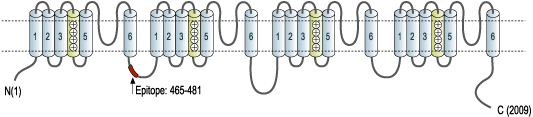
- Peptide (C)TASEHSREPSAAGRLSD, corresponding to amino acid residues 465-481 of rat NaV1.1 (Accession P04774). Intracellular loop between domains I and II.
 Western blot analysis of rat brain membranes:1. Anti-SCN1A (NaV1.1) Antibody (#ASC-001), (1:200).
Western blot analysis of rat brain membranes:1. Anti-SCN1A (NaV1.1) Antibody (#ASC-001), (1:200).
2. Anti-SCN1A (NaV1.1) Antibody, preincubated with SCN1A/Nav1.1 Blocking Peptide (#BLP-SC001).
- Mouse cardiac myocytes (1:500) (Malhotra, J.D. et al. (2001) Circulation 103, 1303.).
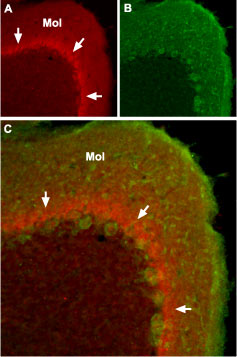 Expression of NaV1.1 sodium channel in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-SCN1A (NaV1.1) Antibody (#ASC-001). A. The distribution of NaV1.1 (red) forms a band (arrows) in the molecular layer (Mol), close to the Purkinje cell bodies. B. Purkinje nerve cells are stained with mouse anti-Parvalbumin (green). C. Confocal merge of NaV1.1 and Parvalbumin.
Expression of NaV1.1 sodium channel in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-SCN1A (NaV1.1) Antibody (#ASC-001). A. The distribution of NaV1.1 (red) forms a band (arrows) in the molecular layer (Mol), close to the Purkinje cell bodies. B. Purkinje nerve cells are stained with mouse anti-Parvalbumin (green). C. Confocal merge of NaV1.1 and Parvalbumin.- Human brain (1:400) (Wang, W. et al. (2011) Brain Res. 1389, 61.).
 Expression of NaV1.1 in rat DRG cellsImmunocytochemical staining of Paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), (1:200), followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. Nuclear staining of cells using the cell-permeable dye Hoechst 33342.
Expression of NaV1.1 in rat DRG cellsImmunocytochemical staining of Paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), (1:200), followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. Nuclear staining of cells using the cell-permeable dye Hoechst 33342.
- Wu, L. et al. (2002) NeuroReport 13, 2547.
- Fang, X. et al. (2002) J. Neurosci. 22, 7425.
- Fjell, J. et al. (2000) NeuroReport 11, 199.
- Baker, M.D. and Wood, J.N. (2001) Trends Pharmacol. Sci. 22, 27.
- Lai, J. et al. (2003) Curr. Opin. Neurobiol. 13, 291.
- Isom, L.L. (2001) Neuroscientist 7, 42.
- Catterall, W.A. et al. (2003) Pharmacol Rev 55, 575.
- Catterall, W.A. et al. (2008) J. Neurosci. 28, 11768.
- Rhodes, T.H. et al. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11147.
Voltage-gated sodium channels (NaV) are essential for the generation of action potentials and for cell excitability1. NaV channels are activated in response to depolarization and selectively allow the flow of Na+ ions. To date, nine NaV α subunits have been cloned and named NaV1.1-NaV1.94-5. The NaV channels are classified into two groups according to their sensitivity to tetrodotoxin (TTX): TTX-sensitive (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6 and NaV1.7) and TTX-resistant (NaV1.5, NaV1.8 and NaV1.9)2-3.
Mammalian sodium channels are heterotrimers composed of a central, pore-forming α subunit and two auxiliary β subunits. The expression of the α subunit isoform is developmentally regulated and tissue specific. Na+ channels in the adult central nervous system and heart contain β1 through β4 subunits, whereas Na+ channels in adult skeletal muscle have only the β1 subunit6,7.
NaV1.1, also referred to as SCN1A, is a tetrodotoxin-sensitive channel and is broadly expressed in neurons7.
Mutations in NaV1.1 are associated with at least two forms of epilepsy. Gain-of-function missense mutations are a primary cause of generalized epilepsy with febrile seizures plus (GEFS+). Loss-of-function mutations cause severe myoclonic epilepsy of infancy (SMEI)8,9.
Application key:
Species reactivity key:
Anti-SCN1A (NaV1.1) Antibody (#ASC-001) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize NaV1.1 from rat, human, and mouse samples.
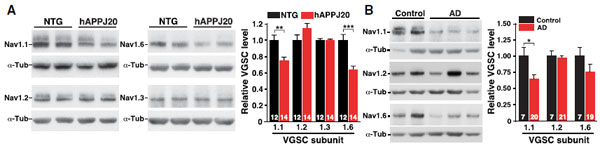 NaV1.1 Levels Decrease in PV Cells of hAPP Mice and in AD Brains.A and B. Western blot analysis of parietal cortex from mice and inferior parietal cortex from humans using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-SCN2A (NaV1.2) Antibody (#ASC-002), Anti-SCN3A (NaV1.3) Antibody (#ASC-004) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). The graphs represent the quantitation of each western blot.Adapted from Verret, L. et al. (2012) with permission of Elsevier.
NaV1.1 Levels Decrease in PV Cells of hAPP Mice and in AD Brains.A and B. Western blot analysis of parietal cortex from mice and inferior parietal cortex from humans using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-SCN2A (NaV1.2) Antibody (#ASC-002), Anti-SCN3A (NaV1.3) Antibody (#ASC-004) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). The graphs represent the quantitation of each western blot.Adapted from Verret, L. et al. (2012) with permission of Elsevier.Applications
Citations
 Multiplex staining of neuronal NaV channels and Ryanodine receptor 2 in mouse cardiomyocytes.Immunocytochemical staining of mouse cardiomyocytes using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-NaV1.5 (SCN5A) (1978-2016) Antibody (#ASC-013) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) antibodies. All three neuronal NaV channels (green) co-localize with Ryanodine receptor 2.
Multiplex staining of neuronal NaV channels and Ryanodine receptor 2 in mouse cardiomyocytes.Immunocytochemical staining of mouse cardiomyocytes using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-NaV1.5 (SCN5A) (1978-2016) Antibody (#ASC-013) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) antibodies. All three neuronal NaV channels (green) co-localize with Ryanodine receptor 2.
Adapted from Radwanski, P.B. et al. (2015) with permission of the European Society of Cardiology. Expression of NaV1.6 increases in rat mECs during epileptogenesis.A. Immunohistochemical of rat mECs using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS. B. Ratio of post-SE and control tissues shows that NaV1.6 expression increases by 46%. C. Somatal expression for NaV1.6 and NaV1.2 (using Anti-SCN2A (NaV1.2) Antibody (#ASC-002)). D. Normalized expression of the channel expression shows that both NaV1.2 and NaV1.6 expression increases in the soma of post-SE tissues. NaV1.1 and NaV1.3 expression, detected using Anti-SCN1A (NaV1.1) Antibody (#ASC-001) and Anti-SCN3A (NaV1.3) Antibody (#ASC-004), respectively, does not change during epileptogenesis.
Expression of NaV1.6 increases in rat mECs during epileptogenesis.A. Immunohistochemical of rat mECs using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS. B. Ratio of post-SE and control tissues shows that NaV1.6 expression increases by 46%. C. Somatal expression for NaV1.6 and NaV1.2 (using Anti-SCN2A (NaV1.2) Antibody (#ASC-002)). D. Normalized expression of the channel expression shows that both NaV1.2 and NaV1.6 expression increases in the soma of post-SE tissues. NaV1.1 and NaV1.3 expression, detected using Anti-SCN1A (NaV1.1) Antibody (#ASC-001) and Anti-SCN3A (NaV1.3) Antibody (#ASC-004), respectively, does not change during epileptogenesis.
Adapted from Hargus, N.J. et al. (2013) with permission of the American Physiological Society. Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.
Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.
Adapted from King, J.H. et al. (2013) with permission of Elsevier.
- Immunohistochemical staining of mouse brain sections. Tested in SCN1A-/- conditional knockout mice.
Kalume, F. et al. (2015) Neurobiol. Dis. 77, 141.
- HEK-293 cell lysate.
Nissenkorn, A. et al. (2019) PLoS ONE 14, e0211901. - Human astrocytoma lysate.
Guan, G. et al. (2018) Neurosci. Lett. 674, 148. - Mouse brain lysate (1:500).
Hamm, V. et al. (2017) Adv. Sci. 3, e1601068. - Mouse brain lysate (1:800).
Hu, T. et al. (2017) Oncotarget 8, 99284. - Rat brain lysate.
Murenzi, E. et al. (2017) Neurotoxicology 60, 260. - Mouse brain lysates.
Liu, C. et al. (2015) J. Biol. Chem. 290, 12048. - Rat pituitary (GH3) cell lysate.
Baroni, D. et al. (2014) Biol. Cell 106, 13. - Rat DRG lysate (1:200).
Cheng, K.I. et al. (2014) Eur. J. Pain 18, 162. - Human and mouse cortical lysates.
Corbett, B.F. et al. (2013) J. Neurosci. 33, 7020. - Human and mouse brain.
Verret, L. et al. (2012) Cell 149, 708. - Mouse heart.
Haufe, V. et al. (2005) J. Physiol 564, 683. - Mouse cardiac myocytes.
Malhotra, J.D. et al. (2001) Circulation 103, 1303. - Mouse brain.
Planells-Cases, R. et al. (2000) Biophys. J. 78, 2878.
- Mouse heart.
Haufe, V. et al. (2005) J. Physiol 564, 683. - Mouse cardiac myocytes.
Malhotra, J.D. et al. (2001) Circulation 103, 1303.
- Human brain sections.
Guan, G. et al. (2018) Neurosci. Lett. 674, 148. - Mouse brain sections.
Martinez-Losa, M. et al. (2018) Neuron 98, 75. - Mouse muscle spindle sections.
Carrasco, D.I. et al. (2017) J. Neurophysiol. 117, 1690. - Rat lumbar spinal cord sections.
Wolff, M. et al. (2016) Neurosci. Res. 109, 16. - Mouse brain sections. Also tested in SCN1A-/- conditional knockout mice.
Kalume, F. et al. (2015) Neurobiol. Dis. 77, 141. - Rat optic nerve.
Sandalon, S. et al. (2013) Exp. Eye Res. 115, 47. - Rat brain sections (1:50).
Qiao, X. et al. (2013) Epilepsy Res. 106, 17. - Rat brain sections (1:250).
Hargus, N.J. et al. (2013) J. Neurophysiol. 110, 1144. - Human and mouse brain.
Verret, L. et al. (2012) Cell 149, 708. - Human brain (1:400).
Wang, W. et al. (2011) Brain Res. 1389, 61. - Mouse heart.
Lei, M. et al. (2004) J. Physiol. 559.3, 835. - Mouse heart.
Malhotra, J.D. et al. (2001) Circulation 103, 1303.
- Mouse ventricular myocytes.
Koleske, M. et al. (2018) J. Gen. Physiol. 150, 991. - Mouse isolated myocytes.
Radwanski, P.B. et al. (2015) Cardiovasc. Res. 106, 143. - Rat microglia (1:400).
Jung, G.Y. et al. (2013) Glia 61, 1807. - Mouse LVA myocytes (1:100).
Ednie, A.R. et al. (2013) J. Mol. Cell. Cardiol. 59, 117. - Mouse heart.
Haufe, V. et al. (2005) J. Physiol 564, 683. - Rat brain.
Black, J.A. et al. (2004) Pain 108, 23. - Mouse heart.
Lei, M. et al. (2004) J. Physiol. 559.3, 835. - Mouse brain.
Planells-Cases, R. et al. (2000) Biophys. J. 78, 2878. - Mouse brain.
Zhou, D. et al. (1998) J. Cell Biol. 143, 1295.
- King, J.H. et al. (2013) Cardiovasc. Res. 99, 751.
