Overview
- Peptide DRLPKSDSEDGPRALNQLS(C), corresponding to amino acid residues 493-511 of rat NaV1.5 (accession number P15389). Intracellular loop between domains I and II.

 Western blot analysis of rat heart membranes:1. Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200).
Western blot analysis of rat heart membranes:1. Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200).
2. Anti-NaV1.5 (SCN5A) (493-511) Antibody, preincubated with Nav1.5/SCN5A (493-511) Blocking Peptide (#BLP-SC005). Western blot analysis of NaV1.5 in NaV1.5 transfected HEK-293 cells:1. Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200).
Western blot analysis of NaV1.5 in NaV1.5 transfected HEK-293 cells:1. Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200).
2. Anti-NaV1.5 (SCN5A) (493-511) Antibody, preincubated with Nav1.5/SCN5A (493-511) Blocking Peptide (#BLP-SC005).- Mouse heart lysate (1:200) (Watanabe, H. et al. (2011) Circulation 124, 1001.).
- Mouse cardiac myocytes (1:500) (Malhotra, J.D. et al. (2001) Circulation 103, 1303.).
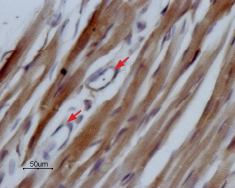 Expression of NaV1.5 in rat heartImmunohistochemical staining of NaV1.5 in rat myocardium paraffin-embedded sections using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:100). Staining is specific for cardiomyocytes while smooth muscles cells in the small vessels are negative (red arrows). Hematoxilin is used as the counterstain.
Expression of NaV1.5 in rat heartImmunohistochemical staining of NaV1.5 in rat myocardium paraffin-embedded sections using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:100). Staining is specific for cardiomyocytes while smooth muscles cells in the small vessels are negative (red arrows). Hematoxilin is used as the counterstain.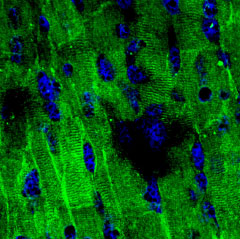 Expression of NaV1.5 in mouse heartImmunohistochemical staining of mouse heart sections using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200). NaV1.5 staining (green) is part of the striate morphology. Nuclei are stained with DAPI (blue).
Expression of NaV1.5 in mouse heartImmunohistochemical staining of mouse heart sections using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), (1:200). NaV1.5 staining (green) is part of the striate morphology. Nuclei are stained with DAPI (blue).
- Neonatal rat ventricular cardiomyocytes (NRCs) (1:200) (Casini, S. et al. (2010) Cardiovasc. Res. 85, 691.).
Voltage-gated Na+ channels (NaV) are responsible for myocardial conduction and maintenance of the cardiac rhythm and are essential for the generation of action potentials and cell excitability.1 Dysfunction or disregulation of cardiac sodium channels can cause several disorders, including cardiac arrhythmias.
The majority of Na+ channels in the mammalian heart are Tetrodotoxin (TTX)-insensitive NaV1.5.2
The putative structure of NaV1.5 consists of four homologous domains (I-IV), each containing six transmembrane segments (S1-S6). Mutations in the C-terminus of NaV1.5 were described in connection to Long QT syndrome and Brugada syndrome.1-2 Recent data have demonstrated selective expression of NaV1.5 in the mouse central nervous system and implicated a role for NaV1.5 in the physiology of the central nervous system.1
Application key:
Species reactivity key:
Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize NaV1.5 sodium channel from rat, human, and mouse samples.
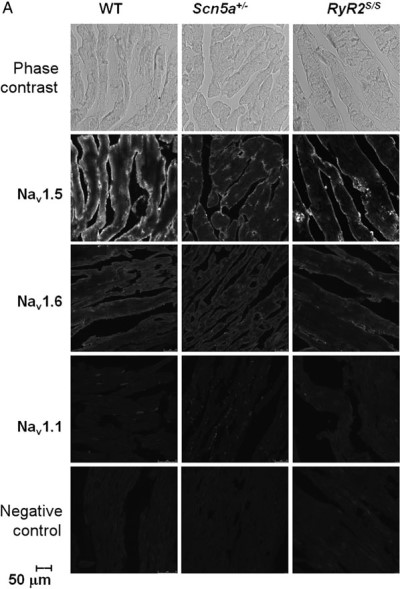 Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.Adapted from King, J.H. et al. (2013) with permission of Elsevier.
Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.Adapted from King, J.H. et al. (2013) with permission of Elsevier.Applications
Citations
- Immunohistochemical staining of mouse lumbar spinal cord sections. Tested in NaV1.5-/- mice.
Pappalardo, L.W. et al. (2018) Glia 66, 2174.
- Rat brain neurolemma lysate.
Murenzi, E. et al. (2017) Neurotoxicology 60, 260. - Mouse neonatal ventricular myocytes.
Xu, Q. et al. (2016) Am. J. Physiol. 311, H1139. - Mouse heart lysate.
Wang, N. et al. (2016) Free Radic. Biol. Med. 96, 34. - Rat heart myoblast (H9C2) cell lysate.
Baroni, D. et al. (2014) Biol. Cell 106, 13. - Mouse muscle lysate.
Boyer, J.G. et al. (2013) Skelet. Muscle 3, 24. - Mouse heart lysate (1:200).
Watanabe, H. et al. (2011) Circulation 124, 1001.
- Mouse neonatal ventricular myocytes.
Xu, Q. et al. (2016) Am. J. Physiol. 311, H1139. - Mouse cardiac myocytes (1:500).
Malhotra, J.D. et al. (2001) Circulation 103, 1303.
- Mouse lumbar spinal cord sections. Also tested in NaV1.5-/- mice.
Pappalardo, L.W. et al. (2018) Glia 66, 2174. - Mouse heart sections.
Chowdhury, R. et al. (2015) Circ. Arrythm. Electrophysiol. 8, 1255.
- Human colon cancer (SW620) cells and HEK-293 transfected cells.
Baptista-Hon, D.T. et al. (2014) Br. J. Anaesth. 113, i39. - Transfected HEK-293 cells (1:300).
Zumhagen, S. et al. (2013) PLoS ONE 8, e67963. - Neonatal rat ventricular cardiomyocytes (NRCs) (1:200).
Casini, S. et al. (2010) Cardiovasc. Res. 85, 691.
- Kim, J.J. et al. (2013) Am. J. Physiol. 304, H848.
- King, J.H. et al. (2013) Cardiovasc. Res. 99, 751.
