Overview
- Peptide CIANHTGVDIHRNGDFQKNG, corresponding to amino acid residues 1042-1061 of rat NaV1.6 (Accession O88420). Intracellular loop between domains II and III.

 Western blot analysis of rat brain membrane:1. Anti-NaV1.6 (SCN8A) Antibody (#ASC-009), (1:200).
Western blot analysis of rat brain membrane:1. Anti-NaV1.6 (SCN8A) Antibody (#ASC-009), (1:200).
2. Anti-NaV1.6 (SCN8A) Antibody, preincubated with Nav1.6/SCN8A Blocking Peptide (#BLP-SC009).
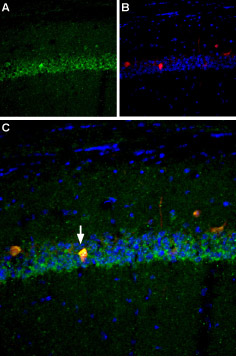 Expression of NaV1.6 in mouse hippocampusImmunohistochemical staining of mouse hippocampus using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). A. NaV1.6 (green) is robustly expressed in the CA1 pyramidal layer (white arrows). B. Staining with mouse anti-parvalbumin (red), a marker of interneurons. C. Merged image of panels A and B reveals that NaV1.6 appears in some interneurons (arrow) but is not restricted to interneurons. DAPI is used as the counterstain.
Expression of NaV1.6 in mouse hippocampusImmunohistochemical staining of mouse hippocampus using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). A. NaV1.6 (green) is robustly expressed in the CA1 pyramidal layer (white arrows). B. Staining with mouse anti-parvalbumin (red), a marker of interneurons. C. Merged image of panels A and B reveals that NaV1.6 appears in some interneurons (arrow) but is not restricted to interneurons. DAPI is used as the counterstain.- Human cervical tissue (1:25) (Hernandez-Plata, E. et al. (2012) Int. J. Cancer 130, 2013.).
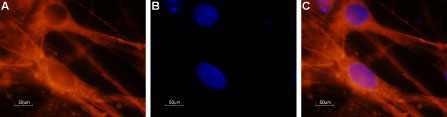 Expression of NaV1.6 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized DRG primary culture. A. Staining using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009), (1:200) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. B. Nuclear staining using the cell-permeable dye Hoechst 33342. C. Merged image of panels A and B.
Expression of NaV1.6 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized DRG primary culture. A. Staining using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009), (1:200) followed by goat anti-rabbit-AlexaFluor-555 secondary antibody. B. Nuclear staining using the cell-permeable dye Hoechst 33342. C. Merged image of panels A and B.- Human cervical cancer cell line (1:25) (Hernandez-Plata, E. et al. (2012) Int. J. Cancer 130, 2013.).
- Wu, L. et al. (2002) NeuroReport 13, 2547.
- Fang, X. et al. (2002) J. Neurosci. 22, 7425.
- Fjell, J. et al. (2000) NeuroReport 11, 199.
- Baker, M.D. and Wood, J.N. (2001) Trends Pharmacol. Sci. 22, 27
- Lai, J. et al. (2003) Curr. Opin. Neurobiol 13, 291.
- Isom, L.L. (2001) Neuroscientist 7, 42.
- Catterall, W.A. et al. (2003) Pharmacol. Rev. 55, 575.
- Catterall, W.A. et al. (2005) Pharmacol. Rev. 57, 397.
- Boiko, T. et al. (2001) Neuron 30, 91.
- Caldwell, J.H. et al. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5616.
- Raman I.M. and Bean B.P. (1997) J. Neurosci. 17, 4517.
Voltage-gated sodium channels (NaV) are essential for the generation of action potentials and for cell excitability.1 NaV channels are activated in response to depolarization and selectively allow flow of Na+ ions. To date, nine NaV α subunits have been cloned and named NaV1.1-NaV1.9.4-5 The NaV channels are classified into two groups according to their sensitivity to tetrodotoxin (TTX): TTX-sensitive (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6 and NaV1.7) and TTX-resistant (NaV1.5, NaV1.8 and NaV1.9).2-3
Mammalian sodium channels are heterotrimers composed of a central, pore-forming α subunit and two auxiliary β subunits. Expression of the α subunit isoform is developmentally regulated and tissue specific. Sodium channels in the adult central nervous system and heart contain β1 through β4 subunits, whereas sodium channels in adult skeletal muscle have only the β1 subunit.6,7
NaV1.6 is highly expressed in the adult brain and localized at high density in Nodes of Ranvier and axon initial segments and at lower density in dendrites and cell bodies of some neurons. NaV1.6 channels are also expressed at high levels in cerebellar Purkinje neurons.8-11
Application key:
Species reactivity key:
Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize NaV1.6 sodium channel from rat, human, and mouse samples.
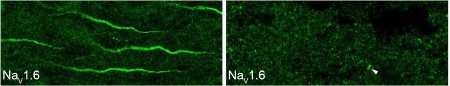
Knockout Validation of Anti-NaV1.6 (SCN8A) Antibody in mouse brain.Immunohistochemical staining of mouse brain sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (green) in neocortex is strongly observed in axonal initial segments. Note that NaV1.6 staining is abolished in brain sections from NaV1.6-/- mice (right panel).Adapted from Tian, C. et al. (2014) Front. Cell. Neurosci. 8, 297. with permission of Frontiers.
Applications
Citations
 Expression of NaV1.6 in rat optic nerveImmunohistochemical staining of rat optic nerve sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (green) is detected at the Nodes of Ranvier. Caspr staining (red) at the paranodes is shown.
Expression of NaV1.6 in rat optic nerveImmunohistochemical staining of rat optic nerve sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (green) is detected at the Nodes of Ranvier. Caspr staining (red) at the paranodes is shown.
Adapted from Arancibia-Carcamo, L.I. et al. (2017) eLife 6, e23329. with permission of eLife Sciences Publications. Expression of NaV1.6 in mouse brainImmunohistochemical staining of mouse brain sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (green) in cortex (upper panels) and hippocampal DG regions (lower panels) is strongly detected in axonal initial segments and co-localizes with AnkG (red).
Expression of NaV1.6 in mouse brainImmunohistochemical staining of mouse brain sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (green) in cortex (upper panels) and hippocampal DG regions (lower panels) is strongly detected in axonal initial segments and co-localizes with AnkG (red).
Adapted from Liu, Y. et al. (2017) Nat. Commun. 8, 355. with permission of Springer Nature. Expression of NaV1.6 in mouse cochleaImmunohistochemical staining of mouse cochlea sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (purple) is localized to the first heminode (upper panels). NaV1.6 staining is strong in Node of Ranvier, including the peri-somatic nodes in the spiral ganglion (SG), (lower panels).
Expression of NaV1.6 in mouse cochleaImmunohistochemical staining of mouse cochlea sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (purple) is localized to the first heminode (upper panels). NaV1.6 staining is strong in Node of Ranvier, including the peri-somatic nodes in the spiral ganglion (SG), (lower panels).
Adapted from Browne, L. et al. (2017) eNeuro 4, e0303. with permission of the Society for Neuroscience. Expression of NaV1.6 in mouse muscle spindles.Immunohistochemical staining of mouse muscle sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (red) is detected in heminodes and sensory terminals of primary endings. A. low magnification. A1a through A1d. High magnifications.
Expression of NaV1.6 in mouse muscle spindles.Immunohistochemical staining of mouse muscle sections using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining (red) is detected in heminodes and sensory terminals of primary endings. A. low magnification. A1a through A1d. High magnifications.
Adapted from Carrasco, D.I. et al. (2017) J. Neurophysiol. 117, 1690. with permission of The American Physiological Society. Multiplex staining of neuronal NaV channels and Ryanodine receptor 2 in mouse cardiomyocytes.Immunocytochemical staining of mouse cardiomyocytes using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-NaV1.5 (SCN5A) (1978-2016) Antibody (#ASC-013) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) antibodies. All three neuronal NaV channels (green) co-localize with Ryanodine receptor 2.
Multiplex staining of neuronal NaV channels and Ryanodine receptor 2 in mouse cardiomyocytes.Immunocytochemical staining of mouse cardiomyocytes using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-NaV1.5 (SCN5A) (1978-2016) Antibody (#ASC-013) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) antibodies. All three neuronal NaV channels (green) co-localize with Ryanodine receptor 2.
Adapted from Radwanski, P.B. et al. (2015) with permission of the European Society of Cardiology.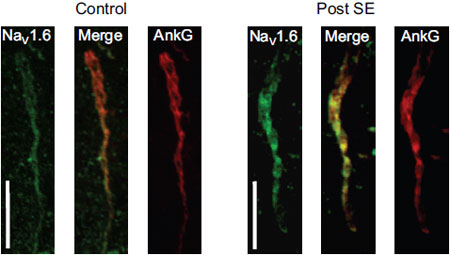 Expression of NaV1.6 increases in rat mECs during epileptogenesisImmunohistochemical of rat medial entorhinal cortex using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS.
Expression of NaV1.6 increases in rat mECs during epileptogenesisImmunohistochemical of rat medial entorhinal cortex using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS.
Adapted from Hargus, N.J. et al. (2013) J. Neurophysiol. 110, 1144. with permission of the American Physiological Society. Expression of NaV1.6 increases in rat mECs during epileptogenesis.A. Immunohistochemical of rat mECs using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS. B. Ratio of post-SE and control tissues shows that NaV1.6 expression increases by 46%. C. Somatal expression for NaV1.6 and NaV1.2 (using Anti-SCN2A (NaV1.2) Antibody (#ASC-002)). D. Normalized expression of the channel expression shows that both NaV1.2 and NaV1.6 expression increases in the soma of post-SE tissues. NaV1.1 and NaV1.3 expression, detected using Anti-SCN1A (NaV1.1) Antibody (#ASC-001) and Anti-SCN3A (NaV1.3) Antibody (#ASC-004), respectively, does not change during epileptogenesis.
Expression of NaV1.6 increases in rat mECs during epileptogenesis.A. Immunohistochemical of rat mECs using Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). NaV1.6 staining is detected in AIS and increases in post-SE tissue. The channel co-localizes with Ankryn-G, a marker of AIS. B. Ratio of post-SE and control tissues shows that NaV1.6 expression increases by 46%. C. Somatal expression for NaV1.6 and NaV1.2 (using Anti-SCN2A (NaV1.2) Antibody (#ASC-002)). D. Normalized expression of the channel expression shows that both NaV1.2 and NaV1.6 expression increases in the soma of post-SE tissues. NaV1.1 and NaV1.3 expression, detected using Anti-SCN1A (NaV1.1) Antibody (#ASC-001) and Anti-SCN3A (NaV1.3) Antibody (#ASC-004), respectively, does not change during epileptogenesis.
Adapted from Hargus, N.J. et al. (2013) with permission of the American Physiological Society. Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.
Decreased expression of NaV1.5 in RyR2s/s atria.Immunohistochemical staining of mouse atria using Anti-NaV1.5 (SCN5A) (493-511) Antibody (#ASC-005), Anti-NaV1.6 (SCN8A) Antibody (#ASC-009) and Anti-SCN1A (NaV1.1) Antibody (#ASC-001). NaV1.5 staining in RyR2s/s atria is lower compared to wild type levels. NaV1.6 staining slightly decreased in RyR2s/s atria. NaV1.1 which is not expressed in the atrial tissue is not detected.
Adapted from King, J.H. et al. (2013) with permission of Elsevier. NaV1.1 Levels Decrease in PV Cells of hAPP Mice and in AD Brains.A and B. Western blot analysis of parietal cortex from mice and inferior parietal cortex from humans using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-SCN2A (NaV1.2) Antibody (#ASC-002), Anti-SCN3A (NaV1.3) Antibody (#ASC-004) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). The graphs represent the quantitation of each western blot.
NaV1.1 Levels Decrease in PV Cells of hAPP Mice and in AD Brains.A and B. Western blot analysis of parietal cortex from mice and inferior parietal cortex from humans using Anti-SCN1A (NaV1.1) Antibody (#ASC-001), Anti-SCN2A (NaV1.2) Antibody (#ASC-002), Anti-SCN3A (NaV1.3) Antibody (#ASC-004) and Anti-NaV1.6 (SCN8A) Antibody (#ASC-009). The graphs represent the quantitation of each western blot.
Adapted from Verret, L. et al. (2012) with permission of Elsevier.
- Rat DRG lysate.
Ding, H.H. et al. (2019) J. Neuroinflammation 16, 29. - Mouse brain lysate.
Anderson, L.L. et al. (2017) Sci. Rep. 7, 1682. - Mouse brain lysate (1:1000).
Hamm, V. et al. (2017) Adv. Sci. 3, e1601068. - Rat trigeminal ganglion lysate.
Yang, K.Y. et al. (2016) J. Dent. Res. 95, 1183. - Rat brain neurolemma lysate.
Murenzi, E. et al. (2016) Neurotoxicology 60, 260. - Rat brain lysate (1:200).
Yin, L. et al. (2015) Cereb. Cortex 27, 509. - Mouse DRG, PN and colon lysates.
Feng, B. et al. (2015) J. Neurophysiol. 113, 2618. - HEK 293 transfected cells (1:100).
Blanchard, M.G. et al. (2015) J. Med. Genet. 52, 330. - Mouse brain lysates.
Liu, C. et al. (2015) J. Biol. Chem. 290, 12048. - Rat DRG lysate (1:200).
Cheng, K.I. et al. (2014) Eur. J. Pain 18, 162. - Immortalized mouse microglial cells (BV-2) (1:500).
Hossain, M.M. et al. (2013) Toxicol. Appl. Pharmacol. 273, 355. - Rat DRG lysates (1:200).
Shen, K.F. et al. (2013) Exp. Neurol. 247, 466. - Human and mouse brain.
Verret, L. et al. (2012) Cell 149, 708. - Mouse brain lysate.
Kearney, J.A. et al. (2002) Hum. Mol. Genet. 11, 2765.
- Rat DRG sections.
Ding, H.H. et al. (2019) J. Neuroinflammation 16, 29. - Rat optic nerve sections (1:500).
Arancibia-Carcamo, L.I. et al. (2017) eLife 6, e23329. - Mouse olfactory tissue sections.
Bolz, F. et al. (2017) Front. Neuroanat. 11, 28. - Mouse cochlea sections.
Browne, L. et al. (2017) eNeuro 4, e0303. - Mouse spindle muscle sections.
Carrasco, D.I. et al. (2017) J. Neurophysiol. 117, 1690. - Mouse brain sections.
Liu, Y. et al. (2017) Nat. Commun. 8, 355. - Mouse brain sections (1:500).
Ottolini, M. et al. (2017) J. Neurosci. 37, 7643. - Rat spinal cord sections (1:200).
Brocard, C. et al. (2016) Nat. Med. 22, 404. - Mouse brain sections.
Valkova, C. et al. (2017) Sci. Rep. 7, 41248. - Mouse brain sections (1:500).
Goebbels, S. et al. (2016) Nat. Neurosci. 20, 10. - Mouse optic nerve sections (1:100).
Stahon, K.E. et al. (2016) J. Neurosci. 36, 9990. - Rat lumbar spinal cord sections.
Wolff, M. et al. (2016) Neurosci. Res. 109, 16. - Rat brain sections.
Zhu, H. et al. (2016) Sci. Rep. 6, 38108. - Mouse L6 DRG and distal colorectum sections (1:1000).
Feng, B. et al. (2015) J. Neurophysiol. 113, 2618. - Mouse, rat and human brain sections. Also tested in NaV1.6-/- mice.
Tian, C. et al. (2014) Front. Cell. Neurosci. 8, 297. - Rat optic nerve.
Sandalon, S. et al. (2013) Exp. Eye Res. 115, 47. - Mouse brain sections (1:500).
Xiao, M. et al. (2013) Moll. Cell. Neurosci. 56, 393. - Rat brain sections (1:200).
Qiao, X. et al. (2013) Epilepsy Res. 106, 17. - Mouse spinal cord and cortex (1:100).
Zoupi, L. et al. (2013) Glia 61, 1236. - Rat brain sections (1:250).
Hargus, N.J. et al. (2013) J. Neurophysiol. 110, 1144. - Rat DRG sections (1:100). Also tested in NaV1.6 siRNA treated animals.
Xie, W. et al. (2013) Pain 154, 1170. - Human cervical tissue (1:25).
Hernandez-Plata, E. et al. (2012) Int. J. Cancer 130, 2013. - Mouse brain sections.
Takano, M. et al. (2012) PLoS ONE 7, e52904. - Mouse DRG sections.
Wittmack, E.K. et al. (2004) J. Neurosci. 24, 6765. - Mouse heart sections.
Maier, S.K. et al. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3507. - Rat heart sections.
Maier, S.K. et al. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3507. - Rat optic nerve tissue.
Jenkins, SM. et al. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 2303.
- Mouse ventricular myocytes.
Koleske, M. et al. (2018) J. Gen. Physiol. 150, 991. - Mouse isolated myocytes.
Radwanski, P.B. et al. (2015) Cardiovasc. Res. 106, 143. - Rat microglia (1:400).
Jung, G.Y. et al. (2013) Glia 61, 1807. - Immortalized mouse microglial cells (BV-2) (1:250).
Hossain, M.M. et al. (2013) Toxicol. Appl. Pharmacol. 273, 355. - Rat DRGs (1:200).
Shen, K.F. et al. (2013) Exp. Neurol. 247, 466. - Human cervical cancer cell line (1:25).
Hernandez-Plata, E. et al. (2012) Int. J. Cancer 130, 2013.
- Baroni, D. et al. (2014) Biol. Cell 106, 13.
- King, J.H. et al. (2013) Cardiovasc. Res. 99, 751.
- Tian, C. et al. (2009) Nature Protocol Exchange. 161.
