Overview
- Peptide (C)KIRYRKASRKSDVTE, corresponding to amino acid residues 701 - 715 of mouse Neogenin (Accession P97798). Extracellular, N-term.
 Western blot analysis of mouse brain membranes (lanes 1 and 3) and rat spleen membranes (lanes 2 and 4):1-2. Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:200).
Western blot analysis of mouse brain membranes (lanes 1 and 3) and rat spleen membranes (lanes 2 and 4):1-2. Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:200).
3-4. Anti-Neogenin (extracellular) Antibody, preincubated with Neogenin (extracellular) Blocking Peptide (BLP-NR195).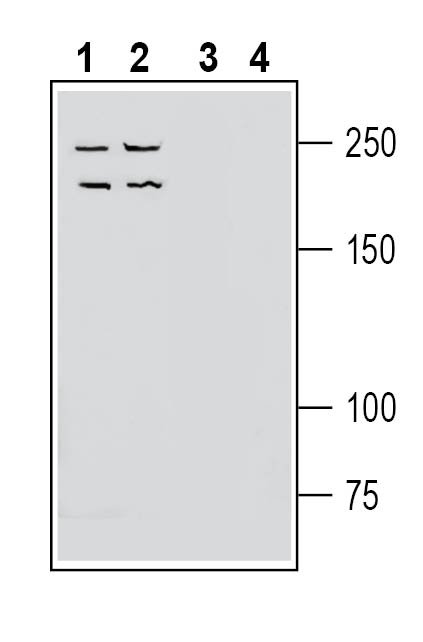 Western blot analysis of human Jurkat T-cell leukemia cell line lysate (lanes 1 and 3) and human LNCaP prostate adenocarcinoma cell line lysate (lanes 2 and 4):1-2. Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:200).
Western blot analysis of human Jurkat T-cell leukemia cell line lysate (lanes 1 and 3) and human LNCaP prostate adenocarcinoma cell line lysate (lanes 2 and 4):1-2. Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:200).
3-4. Anti-Neogenin (extracellular) Antibody, preincubated with Neogenin (extracellular) Blocking Peptide (BLP-NR195).
 Expression of Neogenin in rat parietal cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. Neogenin immunoreactivity (green) appears in cortical neurons in layer 3 (arrows). B. Pre-incubation of the antibody with Neogenin (extracellular) Blocking Peptide (BLP-NR195), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of Neogenin in rat parietal cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-Neogenin (extracellular) Antibody (#ANR-195), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. Neogenin immunoreactivity (green) appears in cortical neurons in layer 3 (arrows). B. Pre-incubation of the antibody with Neogenin (extracellular) Blocking Peptide (BLP-NR195), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Wilson N.H, et al (2008) Int J Biochem Cell Biol., 39, 874.
- Meyerhardt J.A. et al. (1997) Oncogene., 14, 1129.
- Robinson, R.A. et al. (2021) Cell., 15, 184.
- Roth M.P et al. (2021) Blood., 12, 423.
Neogenin also known as NEO1 is a member of the immunoglobulin (Ig) superfamily. It has been reported to be involved in diverse physiology and pathology functions, including cell proliferation, differentiation and migration1. Neogenin was originally isolated from embryonic chicken cerebellum and reported to be homologous to the axon guidance receptor deleted in colorectal cancer (DCC)2.
Neogenin consists of 4 N-terminal Ig-like domains, followed by 6 fibronectin type III-like domains, a single transmembrane helix, and an intracellular domain. Neogenin is capable to mediate attractive and repulsive axon guidance depending on the ligand identity. Two main ligands of neogenin are Netrin-1 (NET1) and repulsive guidance molecules (RGMs)3.
Interaction of Neogenin with RGMs leads to cytoskeleton rearrangements via Rho GTPases, this results in growth cone collapse. In contrast interaction with Netrin-1 triggers attractive growth cone response3. Recently it was shown that Neogenin plays a role in the maintenance of iron homeostasis via interaction with HJV (members of the RGM family)4.
Application key:
Species reactivity key:
Anti-Neogenin (extracellular) Antibody (#ANR-195) is a highly specific antibody directed against an extracellular epitope of the mouse protein. The antibody can be used in western blot, immunohistochemistry and flow cytometry applications. It has been designed to recognize Neogenin from rat, mouse and human samples.

