Overview
- Peptide (C)DLEQMERTVDLKD, corresponding to amino acid residues 189-201 of rat nAChRα2 (Accession P12389). Extracellular, N-terminus.
- Mouse and rat brain membranes. Human SH-SY5Y neuroblastoma cell lysate (1:400-1:1500).
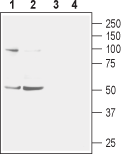 Western blot analysis of mouse (lanes 1 and 3) and rat (lanes 2 and 4) brain membranes:1,2. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody (#ANC-002), (1:400).
Western blot analysis of mouse (lanes 1 and 3) and rat (lanes 2 and 4) brain membranes:1,2. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody (#ANC-002), (1:400).
3,4. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody, preincubated with Nicotinic Acetylcholine Receptor α2/CHRNA2 (extracellular) Blocking Peptide (#BLP-NC002). Western blot analysis of human SH-SY5Y neuroblastoma cell lysate:1. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody (#ANC-002), (1:200).
Western blot analysis of human SH-SY5Y neuroblastoma cell lysate:1. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody (#ANC-002), (1:200).
2. Anti-Nicotinic Acetylcholine Receptor α2 (CHRNA2) (extracellular) Antibody, preincubated with Nicotinic Acetylcholine Receptor α2/CHRNA2 (extracellular) Blocking Peptide (#BLP-NC002).
- Rat free floating brain frozen sections (1:100).
- Live intact rat PC12 pheochromocytoma cells (1:50-1:100).
Nicotinic acetylcholine receptors (nAChRs) mediate the physiological effects of exogenous nicotine. They also play critical physiological roles throughout the brain and body by mediating cholinergic excitatory neurotransmission, modulating the release of neurotransmitters, and have longer-term effects on gene expression and cellular connections1.
nAChRs are pentameric complexes made up of combinations of a number of different nAChR subunits, which can be classified as α subunits, containing two cysteine residues at positions analogous to Cys192 and Cys193, and non-alpha subunits (‘structural’ subunits), which can be defined as β subunits when they are expressed in the vertebrate nervous system2. There are nine α subunits (α2–α10) and three β subunits (β2, β3, and β4) in the CNS3. Nicotinic receptors are assembled as combinations of α (2-6) and and β (2-4) subunits.
All α subunits are expressed in neuronal cells except for the α1 subunit which is specifically expressed in skeletal muscle. They are also expressed in non-neuronal cells such as bronchial epithelial cells4, as well lymphocytes5.
In humans, a mutant α2 subunit has been identified, which forms nAChRs with increased agonist sensitivity and causes a form of familial epilepsy6. Further, an α2 subunit null mouse model has been used to demonstrate a role for α2 nAChR in nicotine-induced modulation of long-term potentiation in the mouse hippocampal CA1 region, which may underlie some of the cognitive effects of nicotine7.
