Overview
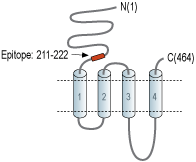
- Peptide (C)NAKGMKGNRREG, corresponding to amino acid residues 211-222 of rat nAChRβ3 (Accession P12391). Extracellular, N-terminus.
 Western blot analysis of rat brain membranes:1. Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody (#ANC-013), (1:400).
Western blot analysis of rat brain membranes:1. Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody (#ANC-013), (1:400).
2. Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody, preincubated with Nicotinic Acetylcholine Receptor β3/CHRNB3 (extracellular) Blocking Peptide (#BLP-NC013).
 Expression of nAChR β3 in live intact rat PC12 cellsCell surface detection of nAChR β3 in live intact rat PC12 pheochromocytoma cells. A. Extracellular staining of cells with Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody (#ANC-013), (1:50), followed by goat anti-rabbit-AlexaFluor-594 secondary antibody (red). B. Live view of the cells. C. Merge image of A and B.
Expression of nAChR β3 in live intact rat PC12 cellsCell surface detection of nAChR β3 in live intact rat PC12 pheochromocytoma cells. A. Extracellular staining of cells with Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody (#ANC-013), (1:50), followed by goat anti-rabbit-AlexaFluor-594 secondary antibody (red). B. Live view of the cells. C. Merge image of A and B.
- Albuquerque, E.X. et al. (2009) Physiol. Rev. 89, 73.
- Karlin, A. et al. (1986) Ann. NY. Acad. Sci. 463, 53.
- Kalamida, D. et al. (2007) FEBS J. 274, 3799.
- Deneris, E.S. et al. (1989) J. Biol. Chem. 264, 6268.
- Dash, B. and Lukas, R.J. (2012) J. Biol. Chem. 287, 14259.
Nicotinic acetylcholine receptors (nAChRs) mediate the physiological effects of exogenous nicotine. They also play critical physiological roles throughout the brain and body by mediating cholinergic excitatory neurotransmission, modulating the release of neurotransmitters, and having longer-term effects on gene expression and cellular connections1.
nAChRs are pentameric complexes made up of combinations of a number of different nAChR subunits. To date, 17 different but related subunits of nAChRs have been identified and cloned. They consist of α subunits (α1-10), which is responsible for the binding of ligands. In fact, this subunit includes a Cys-loop in the first extracellular domain that is required for agonist binding2. The other subunits responsible for making up the active receptor are the β (β1-4), γ, δ and ε subunits3. Structurally, all subunits have the following: a conserved large extracellular N-terminal domain, three conserved transmembrane domains, a variable cytoplasmic loop and a fourth transmembrane domain with a short extracellular C-terminal domain. An active nAChR is generally a heteropentamer of these various subunits organized around a central pore1. The β3 subunit cannot form functional receptors when expressed alone. It only forms operational channels when coexpressed with other functional subunit combinations, and is thus commonly termed ‘orphan’ or ‘auxiliary’ subunits3.
β3 nAChR subunit mRNA is highly expressed in dopaminergic neurons of the substantia nigra and ventral tegmental area4.
α6/β3 nAChRs have been implicated in dopaminergic neurotransmission, nicotine dependence, anxiety, and other important neurophysiological processes5.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope of the rat nAChR β3. Anti-Nicotinic Acetylcholine Receptor β3 (CHRNB3) (extracellular) Antibody (#ANC-013) can be used in western blot and live cell imaging applications. It has been designed to recognize nAChRβ3 from mouse, rat and human samples.
