Overview
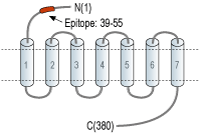
- Peptide (C)NGSVGSEDQQLEPAHIS, corresponding to amino acid residues 39-55 of rat κ-Opioid receptor (Accession P34975). Extracellular, N-terminus.
 Western blot analysis of mouse brain (lanes 1 and 3), rat brain (lanes 2 and 4) and human brain neuroblastoma (SH-SY5Y) cell (lanes 5 and 6) lysates:1,2,5. Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody (#AOR-012), (1:200).
Western blot analysis of mouse brain (lanes 1 and 3), rat brain (lanes 2 and 4) and human brain neuroblastoma (SH-SY5Y) cell (lanes 5 and 6) lysates:1,2,5. Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody (#AOR-012), (1:200).
3,4,6. Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody, preincubated with κ-Opioid Receptor/OPRK1 (extracellular) Blocking Peptide (#BLP-OR012).
 Expression of OPRK1 in rat piriform cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody (#AOR-012), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. OPRK1 immunoreactivity (green) appears in cells and processes (arrows) of the piriform cortex. B. Pre-incubation of the antibody with κ-Opioid Receptor/OPRK1 (extracellular) Blocking Peptide (#BLP-OR012), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of OPRK1 in rat piriform cortex.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody (#AOR-012), (1:300), followed by goat anti-rabbit-AlexaFluor-488. A. OPRK1 immunoreactivity (green) appears in cells and processes (arrows) of the piriform cortex. B. Pre-incubation of the antibody with κ-Opioid Receptor/OPRK1 (extracellular) Blocking Peptide (#BLP-OR012), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Pan, L. et al. (2005) Neuroscience 133, 209.
- IUPHAR
- Matthes, H.W. et al. (1998) J. Neurosci. 18, 7285.
- Di Chiara, G. and North, R.A. (1992) Trends Pharmacol. Sci. 13, 185.
- Schwarzer, C. (2009) Pharmacol. Ther. 123, 353.
Endogenous opiates such as endorphins, endomorphins, and enkephalins, as well as opiate drugs (including morphine) exert their effects by binding to opioid receptors. Three "classic" types of opioid receptors have been identified: mu (µ)-opioid (MOP) receptor, delta (δ)-opioid (DOP) receptor, and kappa (κ)-opioid (KOP) receptor1. Recently, the nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor was also described. Despite its significant sequence homology, its pharmacological profile differs greatly from those of the classic µ, δ, and κ receptors2.
The opioid receptors belong to the G protein-coupled receptor (GPCR) superfamily whose members share a common structure of seven putative transmembrane domains, an extracellular amino terminus, a cytoplasmic carboxyl terminus, and a third intracellular loop important for binding G proteins1.
All three classic opioid receptors mediate opioid-induced analgesia. Supraspinal analgesia is mainly mediated by the µ-opioid receptor, whereas µ-, δ-, and κ-receptors participate in the control of pain at the spinal level3. The opioid receptors also mediate the mood-altering properties of opioids4.
κ-Opioid Receptor is activated by the endogenous peptide dynorphin. Malfunction of their signaling may be involved in epilepsy, addiction, depression, schizophrenia, and chronic pain5.
κ-Opioid Receptor is localized to a number of regions in the brain, as well as the spinal cord, the enteric nervous system and the digestive system5.
Application key:
Species reactivity key:
Alomone Labs is pleased to offer a highly specific antibody directed against an epitope of the rat κ-opioid receptor. Anti-κ-Opioid Receptor (OPRK1) (extracellular) Antibody (#AOR-012) can be used in western blot analysis. The antibody recognizes an extracellular epitope and thus is ideal for detecting the receptor in living cells. The antibody was designed to recognize κ-opioid receptor from mouse, rat and human samples.

