Overview
- Peptide CRPIYEFHGLYEEK, corresponding to amino acid residues 270-283 of human P2X1 receptor (Accession P51575). Extracellular loop.

 Western blot analysis of rat brain (lanes 1 and 3) and mouse brain (lanes 2 and 4) lysates:1,2. Anti-P2X1 Receptor (extracellular) Antibody (#APR-022), (1:200).
Western blot analysis of rat brain (lanes 1 and 3) and mouse brain (lanes 2 and 4) lysates:1,2. Anti-P2X1 Receptor (extracellular) Antibody (#APR-022), (1:200).
3,4. Anti-P2X1 Receptor (extracellular) Antibody, preincubated with P2X1 Receptor (extracellular) Blocking Peptide (#BLP-PR022).
 Expression of P2X1 Receptor in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-P2X1 Receptor (extracellular) Antibody (#APR-022). A. Most of P2RX1 labeling (green) appears in fine processes in the molecular layer (MOL) and in Purkinje cells (arrows show examples). B. DAPI is used as the counterstain (blue). C. Merge of A and B.
Expression of P2X1 Receptor in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-P2X1 Receptor (extracellular) Antibody (#APR-022). A. Most of P2RX1 labeling (green) appears in fine processes in the molecular layer (MOL) and in Purkinje cells (arrows show examples). B. DAPI is used as the counterstain (blue). C. Merge of A and B.
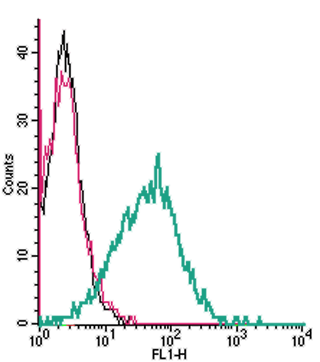 Cell surface detection of P2X1 by indirect flow cytometry in live intact human MEG-01 megakaryocytic leukemia cells:___ Cells.
Cell surface detection of P2X1 by indirect flow cytometry in live intact human MEG-01 megakaryocytic leukemia cells:___ Cells.
___ Cells + goat-anti-rabbit-FITC.
___ Cells + Anti-P2X1 Receptor (extracellular) Antibody (#APR-022), (2.5μg) + goat-anti-rabbit-FITC.- The blocking peptide is not suitable for this application.
 Expression of P2X1 Receptor in human SH-SYS5 cellsCell surface detection of P2X1 Receptor in intact living human SH-SYS5 cells. A. Extracellular staining of cells with Anti-P2X1 Receptor (extracellular) Antibody (#APR-022), (1:50) followed by goat anti-rabbit-AlexaFluor-488 secondary antibody. B. Nuclear staining DAPI as the counterstain. C. Merged images of A and B.
Expression of P2X1 Receptor in human SH-SYS5 cellsCell surface detection of P2X1 Receptor in intact living human SH-SYS5 cells. A. Extracellular staining of cells with Anti-P2X1 Receptor (extracellular) Antibody (#APR-022), (1:50) followed by goat anti-rabbit-AlexaFluor-488 secondary antibody. B. Nuclear staining DAPI as the counterstain. C. Merged images of A and B.
- Prasad, M. et al. (2001) J. Physiol. 537, 667.
- Florenzano, F. et al. (2002) Neuroscience 115, 425.
- Ashcroft, F.M. et al. (2000) Ion Channels and Disease Ed 1, p. 405, Academic Press, San Diego.
- Khakh, B.S. et al. (2001) Pharmacol. Rev. 53, 107.
- Ding, Y. et al. (2000) J. Auton. Nerv. Syst. 81, 289.
- Lê, K.T. et al. (1998) J. Neurosci. 18, 7152.
- Robertson, S.J. et al. (2001) Curr. Opin. Neurobiol. 11, 378.
- Dunn, P.M. et al. (2001) Prog. Neurobiol. 65, 107.
- Kim, M. et al. (2001) EMBO J. 20, 6347.
- Burnstock, G. (2001) Trends Pharmacol. Sci. 22, 182.
- Oury, C. et al. (2003) Blood 101, 3969.
The P2X receptors belong to the ligand-gated ion channel family and are activated by extracellular ATP.
The structure and function of the P2X receptors, investigated mainly using in vitro models, indicate their involvement in synaptic communication, cell death, and differentiation.
Seven mammalian P2X receptor subtypes (P2X1–P2X7) have been identified and cloned1-3. All P2X receptor subtypes share the same structure of intracellular N- and C-termini two membrane-spanning domains and a large extracellular loop.
All P2X receptor subtypes can assemble to form homomeric or heteromeric functional channels with the exception of P2X6, which only seems to function as part of a heteromeric complex4-9.
The various P2X receptor subtypes show distinct expression patterns. P2X1-6 have been found in the central and peripheral nervous systems, while the P2X7 receptor is predominantly found in cells of the immune system4. The P2X1 receptor is present in smooth muscle, cerebellum, dorsal horn spinal neurons, and platelets where it is suggested to play a regulatory role during in vivo homeostasis and thrombosis3,4,10,11.
Application key:
Species reactivity key:
Anti-P2X1 Receptor (extracellular) Antibody (#APR-022) is a highly specific antibody directed against an epitope of the human protein. The antibody can be used in western blot, immunocytochemistry, immunohistochemistry, and indirect flow cytometry applications. It has been designed to recognize P2X1 receptor from rat, mouse, and human samples.
Applications
Citations
- Human eosinophils.
Wright, A. et al. (2016) J. Immunol. 196, 4877.
