Overview
- Peptide (C)KLKHPVMSVSYKEVDR, corresponding to amino acid residues 95 - 110 of mouse PACC1 (Accession Q9D771). Extracellular loop.
PACC1/TMEM206 (extracellular) Blocking Peptide (BLP-CL031)
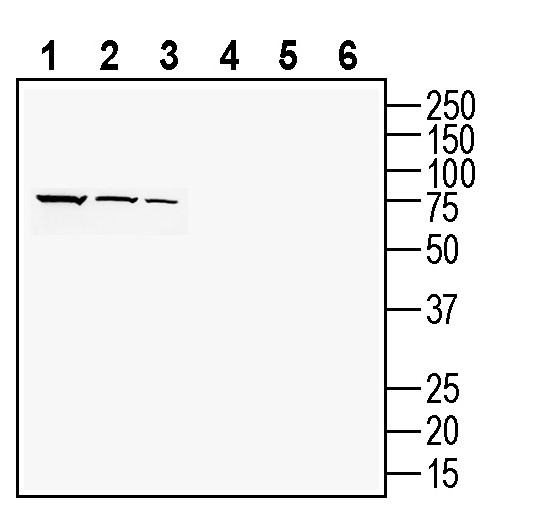 Western blot analysis of rat brain lysate (lanes 1 and 4), mouse brain membranes (lanes 2 and 5) and rat kidney lysates (lanes 3 and 6):1-3. Anti-PACC1/TMEM206 (extracellular) Antibody (#ACL-031), (1:200).
Western blot analysis of rat brain lysate (lanes 1 and 4), mouse brain membranes (lanes 2 and 5) and rat kidney lysates (lanes 3 and 6):1-3. Anti-PACC1/TMEM206 (extracellular) Antibody (#ACL-031), (1:200).
4-6. Anti-PACC1/TMEM206 (extracellular) Antibody, preincubated with PACC1/TMEM206 (extracellular) Blocking Peptide (BLP-CL031).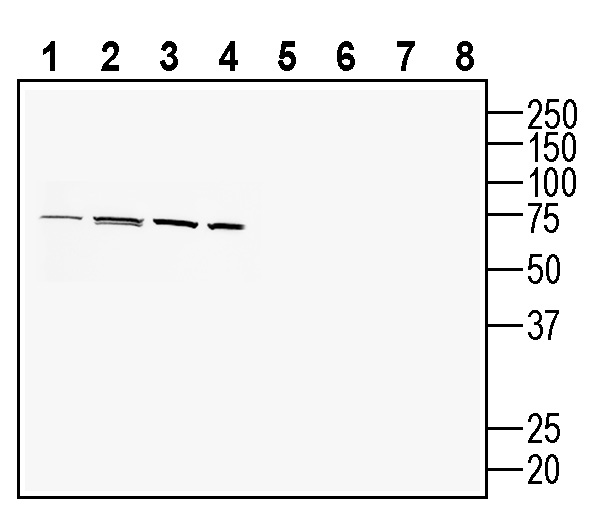 Western blot analysis of human U-87 MG glioblastoma cell line lysate (lanes 1 and 5), human HT-29 colon adenocarcinoma cell line lysate (lanes 2 and 6), human THP-1 monocytic leukemia cell line lysate (lanes 3 and 7) and mouse BV-2 microglia cell line lysate (lanes 4 and 8):1-4. Anti-PACC1/TMEM206 (extracellular) Antibody (#ACL-031), (1:200).
Western blot analysis of human U-87 MG glioblastoma cell line lysate (lanes 1 and 5), human HT-29 colon adenocarcinoma cell line lysate (lanes 2 and 6), human THP-1 monocytic leukemia cell line lysate (lanes 3 and 7) and mouse BV-2 microglia cell line lysate (lanes 4 and 8):1-4. Anti-PACC1/TMEM206 (extracellular) Antibody (#ACL-031), (1:200).
5-8. Anti-PACC1/TMEM206 (extracellular) Antibody, preincubated with PACC1/TMEM206 (extracellular) Blocking Peptide (BLP-CL031).
The proton-activated Cl- (PAC) channel, which opens by extracellular acidic pH, has been well characterized in several cells and tissues by electrophysiology recordings, although its molecular identity was unknown.
Recently, two independent groups, identified a two-transmembrane protein named PACC1 (or TMEM206) as the molecular underlie of the proton-activated Cl- channel 1,2.
PACC1 has no discernible sequence homology with previously characterized Cl- channels, although its structure of two transmembrane domains with cytoplasmic N- and C-termini and a large extracellular loop, resembles that of the Na+ -selective channels ASICs and ENaCs. In fact, recent work suggests that the functional unit of the proton-activated Cl- channel is a trimeric form of PACC1 subunits3,4.
PAC activity that is stimulated by the lowering of extracellular pH and has been recorded in a wide range of mammalian cells and tissues. Acidic pH is crucial for the function of intracellular organelles in the secretory and endocytic pathways and is also one of the pathological hallmarks of many diseases, including cerebral and cardiac ischemia, cancer, infection and inflammation. Therefore, it is no surprise that the involvement of PACC1 in several physiological processes of the endocytic pathway5,6 and pathological conditions such as acid-induced cell death and brain damage after ischemic stroke7 or that upregulation of PACC1 is associated with poor prognosis in patients with hepatocellular carcinoma8.
