Overview
- Peptide (C)KDYGVSEYRTVQRGK, corresponding to amino acid residues 541 - 555 of mouse SLC15A2 (Accession Q9ES07). Extracellular, 5th loop.
 Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain membranes (lanes 2 and 4):1-2. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:500).
Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain membranes (lanes 2 and 4):1-2. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:500).
3-4. Anti-PepT2/SLC15A2 (extracellular) Antibody, preincubated with PepT2/SLC15A2 (extracellular) Blocking Peptide (BLP-PT002). Western blot analysis of rat kidney lysate:1. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:200).
Western blot analysis of rat kidney lysate:1. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:200).
2. Anti-PepT2/SLC15A2 (extracellular) Antibody, preincubated with PepT2/SLC15A2 (extracellular) Blocking Peptide (BLP-PT002).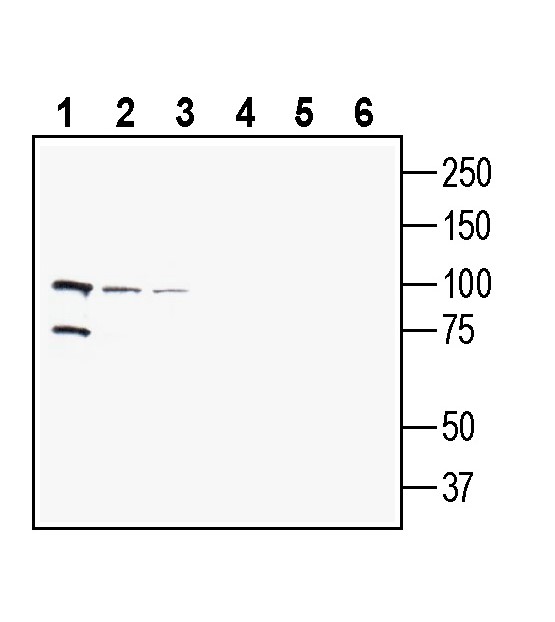 Western blot analysis of human MCF-7 breast adenocarcinoma cell line lysate (lanes 1 and 4), human THP-1 monocytic leukemia cell line lysate (lanes 2 and 5) and human SH-SY5Y neuroblastoma cell line lysate (lanes 3 and 6):1-3. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:200).
Western blot analysis of human MCF-7 breast adenocarcinoma cell line lysate (lanes 1 and 4), human THP-1 monocytic leukemia cell line lysate (lanes 2 and 5) and human SH-SY5Y neuroblastoma cell line lysate (lanes 3 and 6):1-3. Anti-PepT2/SLC15A2 (extracellular) Antibody (#APT-002), (1:200).
4-6. Anti-PepT2/SLC15A2 (extracellular) Antibody, preincubated with PepT2/SLC15A2 (extracellular) Blocking Peptide (BLP-PT002).
Members of the proton-coupled oligopeptide transporter (POT) family are integral membrane proteins that consist of 12 transmembrane helices, having both the amino- and carboxy-terminus localized intracellularly. Four members of the POT family, called peptide transporter 1 (PEPT1, encoded by SLC15A1), peptide transporter 2 (PEPT2, encoded by SLC15A2), peptide/histidine transporter 1 (PHT1, encoded by SLC15A4) and peptide/histidine transporter 2 (PHT2, encoded by SLC15A3), have been identified in humans and other mammals1,2. They transport a wide range of di- and tripeptides and peptide-like drugs via an inwardly-directed proton gradient. PHT1 and PHT2 are also able to transport the free amino acid histidine across biological membranes3. PEPT1 was characterized as a high-capacity, low-affinity transporter4 and is expressed especially in the small intestine, where it facilitates the luminal uptake of di- and tripeptides, as well as peptidomimetics and was also found in placenta, liver, kidney, and pancreas5. Additionally, PEPT1 is suggested to mediate intracellular transport, as it was found in nuclei and lysosomes6. In contrast to PEPT1, PEPT2 was characterized as a high-affinity, low-capacity transporter7 and found to be expressed especially in kidney, responsible for the renal reabsorption of filtered di- and tripeptides, as well as peptide-like agents8. In addition, this transporter is present in brain, here with a particular role in peptide uptake out of the cerebrospinal fluid into the choroid plexus9, lung10, skin11 and mammary gland12. PEPT2 had significant expression in the choroid plexus epithelial cells13. The choroid plexus acts as a barrier between the blood and the cerebrospinal fluid (CSF) which surrounds the brain and contains neuronal nutrients and waste. PEPT2 has been implicated in the clearance of peptides from the CSF, acting as an efflux pump14,15 and ensuring CSF homoeostasis16,17. The expression of PEPT2 in the choroid plexus and the lung highlight the versatility of the transporters and the necessity for a deeper understanding of the transporters to assess the factors which influence their activity. This is also highly relevant to medicine, as PEPT1 and PEPT2 have been implicated in the transport and retention of peptidomimetic drugs18.
PEPT2 expression has been observed in the lung10,12,19-22. Lung epithelial tissue was shown to contain PEPT2 messenger ribonucleic acid (mRNA), and white lung preparations are able to transport fluorophore conjugated peptide, indicating functional PEPT2 expression. It is not clear what the primary function of PEPT2 is in the lung. It is a possibility that PEPT2 functions as part of the innate immune system23.
