Overview
- Peptide (C)KLRGARYQPGAGLRAD, corresponding to amino acid residues 24-39 of mouse S1PR5 (EDG8) (Accession Q91X56). Extracellular, N-terminus.
- Rat cortex, cerebellum, hippocampus and mouse brain lysates (1:200-1:1000).
 Western blot analysis of rat cortex (lanes 1 and 5), rat cerebellum (lanes 2 and 6), rat hippocampus (lanes 3 and 7) and mouse brain (lanes 4 and 8) lysates:1-4. Anti-S1PR5/EDG8 (extracellular) Antibody (#ASR-015), (1:200).
Western blot analysis of rat cortex (lanes 1 and 5), rat cerebellum (lanes 2 and 6), rat hippocampus (lanes 3 and 7) and mouse brain (lanes 4 and 8) lysates:1-4. Anti-S1PR5/EDG8 (extracellular) Antibody (#ASR-015), (1:200).
5-8. Anti-S1PR5/EDG8 (extracellular) Antibody, preincubated with S1PR5/EDG8 (extracellular) Blocking Peptide (#BLP-SR015).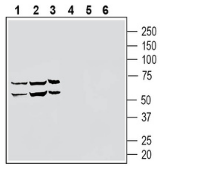 Western blot analysis of human THP-1 monocytic leukemia cell line lysate (lanes 1 and 4), human NK-92 natural killer cell line lysate (lanes 2 and 5) and human HL-60 promyelocytic leukemia cell line lysate (lanes 3 and 6) lysates:1-3. Anti-S1PR5/EDG8 (extracellular) Antibody (#ASR-015), (1:200).
Western blot analysis of human THP-1 monocytic leukemia cell line lysate (lanes 1 and 4), human NK-92 natural killer cell line lysate (lanes 2 and 5) and human HL-60 promyelocytic leukemia cell line lysate (lanes 3 and 6) lysates:1-3. Anti-S1PR5/EDG8 (extracellular) Antibody (#ASR-015), (1:200).
4-6. Anti-S1PR5/EDG8 (extracellular) Antibody, preincubated with the negative control antigen.
Sphingosine-1-phosphate receptor 5 also known as S1PR5 (EDG8) is a G protein-coupled receptor which binds the lipid signaling molecule sphingosine 1-phosphate (S1P).
Lysophospholipids encompass a broad range of small, membrane-derived phospholipids that act as extracellular signals, mediated through specific receptors1. S1PR5 is a high-affinity cell surface S1P receptor, a member of the S1PR family. Like all GPCRs it has the classical structure of seven transmembrane domains, an extracellular N-terminus and a cytosolic C-terminal1.
The signaling pathways triggered by S1P/S1P binding play a critical regulatory role in many pathophysiological processes, including skeletal muscle and nervous system degeneration, inflammation, cancer, and autoimmune disorders and hence this interaction has important implications for clinical applications and for personalized medicine2,3. Targeting S1P receptors and their downstream signaling cascades is considered as a potential treatment for cancer and various autoimmune diseases4.
