Overview
- Peptide (C)RSPEKQLHPAGATIH, corresponding to amino acid residues 849 - 863 of human SEZ6 (Accession Q53EL9). Extracellular, N-terminus
SEZ6 (extracellular) Blocking Peptide (#BLP-NR206)
 Western blot analysis of rat brain lysates (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Anti-SEZ6 (extracellular) Antibody (#ANR-206), (1:200).
Western blot analysis of rat brain lysates (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Anti-SEZ6 (extracellular) Antibody (#ANR-206), (1:200).
3-4. Anti-SEZ6 (extracellular) Antibody, preincubated with SEZ6 (extracellular) Blocking Peptide (BLP-NR206).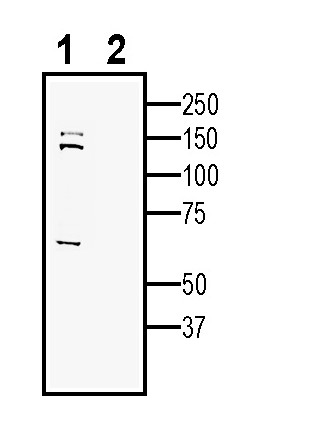 Western blot analysis of human SH-SY5Y neuroblastoma cell line lysate:1. Anti-SEZ6 (extracellular) Antibody (#ANR-206), (1:200).
Western blot analysis of human SH-SY5Y neuroblastoma cell line lysate:1. Anti-SEZ6 (extracellular) Antibody (#ANR-206), (1:200).
2. Anti-SEZ6 (extracellular) Antibody, preincubated with SEZ6 (extracellular) Blocking Peptide (BLP-NR206).
Seizure protein 6 homolog, SEZ6, is a member of the SEZ6 protein family, which are expressed widely throughout the brain and play a role in synaptic development and function, cell-cell recognition, and neuronal membrane signaling. SEZ6 is important for the achievement of the necessary balance between dendrite elongation and branching during the elaboration of a complex dendritic arbor, as well as being involved in the development of appropriate excitatory synaptic connectivity.1
SEZ6 exists in three splice isoforms: two different transmembrane protein isoforms and a truncated secreted isoform. SEZ6 transmembrane and secreted isoforms perform opposing actions on dendritic outgrowth in vitro; overexpression of the full-length transmembrane SEZ6 inhibits outgrowth, while secreted SEZ6 promotes it. BACE1 has been shown to cleave the transmembrane form of SEZ6 to produce a shed ectodomain similar to the secreted SEZ6 isoform.
SEZ6 family proteins were shown to contribute to the refinement of synaptic connectivity between climbing fibers and Purkinje cells (PCs) in the cerebellum of triple knockout (KO) mice lacking all three SEZ6 family members, as SEZ6 KO neurons failed to achieve a mature state of mono-innervation of PCs by climbing fibers, which resulted in significant motor coordination deficits. Neurons of KO mice exhibit morphological alterations, including an increased number of dendrites and reduced dendritic spine densities, altered electrophysiological properties, including reduced postsynaptic responses of cortical neurons, and memory, hippocampal-dependent learning, and motor deficits.2
SEZ6 is required for normal neuronal development and function, particularly for dendrite and spine development. High levels of SEZ6 are found in the developing and postnatal forebrain, and its expression remains relatively high in regions of the adult mouse cortex, hippocampus, striatum, olfactory tubercule, retina and spinal cord. SEZ6 is localized to the somatodendritic compartment of neurons and is upregulated by neuronal activity. SEZ6 gene mutations and altered expression have been associated with febrile seizures, autism spectrum disorder (ASD), intellectual disability, developmental delay, and childhood-onset schizophrenia. Similarly, elevated levels of SEZ6 in the cerebrospinal fluid (CSF) are observed in adult patients with psychiatric disorders.3
Interestingly, SEZ6 has been identified as a target for antibody-drug conjugate (ADC) therapy as it is abundantly expressed on the surface of neuroendocrine tumors and tissues, including small-cell lung cancer and small cell carcinoma of the ovary. Due to its minimal expression in normal tissues and rapid internalization upon antibody binding, it is a promising candidate for an effective and safe ADC-based therapeutic approach.4
