Overview
- Peptide (C)GSAYKLKSYTEGYGK, corresponding to amino acid residues 101 - 115 of mouse SLC38A9 (Accession Q8BGD6). Intracellular, N-term.
SLC38A9 Blocking Peptide (#BLP-NT189)
 Western blot analysis of rat brain membrane (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Anti-SLC38A9 Antibody (#ANT-189), (1:200).
Western blot analysis of rat brain membrane (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Anti-SLC38A9 Antibody (#ANT-189), (1:200).
3-4. Anti-SLC38A9 Antibody, preincubated with SLC38A9 Blocking Peptide (#BLP-NT189).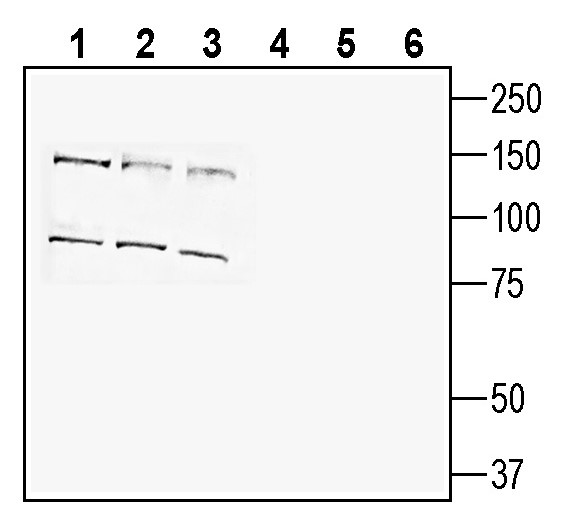 Western blot analysis of human HepG2 hepatocellular carcinoma cell line lysate (lanes 1 and 4), human MCF-7 breast adenocarcinoma cell line lysate (lanes 2 and 5) and human THP-1 monocytic leukemia cell line lysate (lanes 3 and 6):1-3. Anti-SLC38A9 Antibody (#ANT-189), (1:200).
Western blot analysis of human HepG2 hepatocellular carcinoma cell line lysate (lanes 1 and 4), human MCF-7 breast adenocarcinoma cell line lysate (lanes 2 and 5) and human THP-1 monocytic leukemia cell line lysate (lanes 3 and 6):1-3. Anti-SLC38A9 Antibody (#ANT-189), (1:200).
4-6. Anti-SLC38A9 Antibody, preincubated with SLC38A9 Blocking Peptide (#BLP-NT189).
Sodium-coupled neutral amino acid transporter 9 (SLC38A9) is a lysosomal amino acid transporter, involved in the regulation of mechanistic target of rapamycin complex 1 (mTORC1) activity in response to amino acid levels within the lysosomal lumen1,2. A sodium-couple amino acid transporter at the lysosome, SLC38A9, senses arginine from within the lysosome to convey arginine sufficiency to mTORC1.
SLC38A9 forms a complex with other lysosomal resident proteins including the v-ATPase and a pentameric complex called Ragulator3-5. v-ATPase function is necessary for mTORC1 activation, but the molecular mechanism of this regulation is currently unclear2. Ragulator binds strongly to the Rag GTPases and was shown to function as a guanine exchange factor (GEF) for RagA/B6,7. Potentially SLC38A9 and the v-ATPase are able to regulate mTORC1 activity by modulating Ragulator’s GEF activity. RagA/B is not the only Rag GTPase that is regulated, but a Folliculin-FNIP2 complex is a GAP for RagC/D8,9.
In short, SLC38A9 is a functional component of the lysosomal amino acid sensing machinery involved in the control of mTORC1 activity, that underlies the regulation of the metabolic status and cellular responses to growth factors, energy, glucose and amino acid levels 4.
