Overview
- Peptide (C)KHWGRRKAW(S)RQLGE, corresponding to amino acid residues 42-56 of mouse TREM2 (Accession Q99NH8). Extracellular, N-terminus.
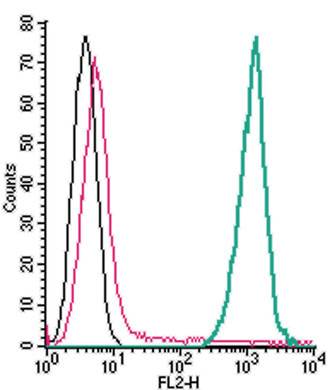 Cell surface detection of TREM2 by direct flow cytometry in live intact mouse J774 macrophage cells:___ Cells.
Cell surface detection of TREM2 by direct flow cytometry in live intact mouse J774 macrophage cells:___ Cells.
___ Cells + Rabbit IgG isotype control-PE.
___ Cells + Anti-TREM2 (extracellular)-PE Antibody (#ANR-018-PE), (2.5µg). Cell surface detection of TREM2 by direct flow cytometry in live intact mouse BV-2 microglia cells:___ Cells.
Cell surface detection of TREM2 by direct flow cytometry in live intact mouse BV-2 microglia cells:___ Cells.
___ Cells + Rabbit IgG isotype control-PE.
___ Cells + Anti-TREM2 (extracellular)-PE Antibody (#ANR-018-PE), (2.5µg). Cell surface detection of TREM2 by direct flow cytometry in live intact human THP-1 monocytic leukemia cells:___ Cells.
Cell surface detection of TREM2 by direct flow cytometry in live intact human THP-1 monocytic leukemia cells:___ Cells.
___ Cells + Rabbit IgG isotype control-PE.
___ Cells + Anti-TREM2 (extracellular)-PE Antibody (#ANR-018-PE), (2.5µg). Cell surface detection of TREM2 by direct flow cytometry in live intact mouse SIM-A9 microglia cells:___ Cells.
Cell surface detection of TREM2 by direct flow cytometry in live intact mouse SIM-A9 microglia cells:___ Cells.
___ Cells + Rabbit IgG isotype control-PE (#RIC-001-PE).
___ Cells + Anti-TREM2 (extracellular)-PE Antibody (#ANR-018-PE), (5µg).
TREM2 (triggering receptor expressed on myeloid cells-2) is a type I transmembrane microglial surface receptor that is found to be expressed on myeloid cells including monocyte-derived dendritic cells, osteoclasts and bone-marrow derived macrophage. TREM2 protein transduces intracellular signals that regulate microglial functions such as cytokine production, migration, proliferation, phagocytosis, cell survival, synapse elimination, and compaction of amyloid plaques1,2.
The TREM2 structure includes an immunoglobulin-like extracellular domain responsible for ligand binding, a transmembrane region and a short cytoplasmatic tail. The protein also includes an ectodomain that binds anionic and zwitterionic lipids, apolipoproteins and lipoprotein particles2,3.
Studies show that TREM2 knockout mice display decreased levels of inflammatory cytokines. TREM2 has been shown to control two signaling pathways in the brain: regulation of phagocytosis and suppression of inflammatory reactivity. Nasu-Hakola disease, a rare and fatal disease, and polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy has been found to be linked to homozygous loss-of-function mutations in TREM2 protein1,2. Studies also show that variants of TREM2 are associated with Alzheimer’s disease2,3.
