Overview
- Peptide (C)GHFLKEPFPESTD, corresponding to amino acid residues 393-405 of rat TrkC (Accession Q03351). Extracellular, N-terminus.
- HEK-TrkC transfected cells (1:400).
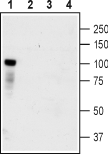 Western blot analysis of HEK-TrkC transfected cells (lanes 1 and 3) and HEK untransfected cells (lanes 2 and 4) lysates:1,2. Anti-TrkC (extracellular) Antibody (#ANT-020), (1:400).
Western blot analysis of HEK-TrkC transfected cells (lanes 1 and 3) and HEK untransfected cells (lanes 2 and 4) lysates:1,2. Anti-TrkC (extracellular) Antibody (#ANT-020), (1:400).
3,4. Anti-TrkC (extracellular) Antibody, preincubated with TrkC (extracellular) Blocking Peptide (#BLP-NT020).
- Rat dorsal root ganglia (1:100).
- Rat live PC12 pheochromocytoma cells (1:50).
BDNF and NT-4 belong to the neurotrophin family which also includes NGF and NT-3. These neurotrophins bind two groups of receptors. The p75NTR receptor is common to all four neurotrophins and is a member of the tumor necrosis factor receptor family. The tropomyosin-related kinase (Trk) receptors are receptor tyrosine kinases (RTKs) and three receptors form this family: TrkA, TrkB, and TrkC1.
As mentioned above, the p75NTR receptor binds to all neurotrophins with similar affinities while the Trk receptors are the ones to display the selectivity for the neurotrophins. TrkA is activated by NGF binding, TrkB by that of BDNF and NT-4, while TrkC is stimulated by the binding of NT-3 1.
All three Trk receptors are highly expressed in the mammalian brain in very distinct regions and are also expressed in the peripheral nervous system2-4. Cholinergic neurons in the basal forebrain exclusively express TrkA, while TrkB and TrkC are highly expressed in the hippocampus. Motor and sensory neurons in the peripheral nervous system express Trk receptors. Interestingly, Trk receptors are not essential for development, but knockout mice die shortly after birth. Indeed, TrkB-deficient mice demonstrate a significant decrease in motor neurons and synaptogenesis1.
Trk receptors have many motifs in the extracellular region, including cell-adhesion domains, three tandem leucine rich motifs flanked by two clusters of cysteines. In the membrane proximal region of the receptor there are also two immunoglobulin-like domains5. The binding of neurotrophins to Trk receptors promotes receptor dimerization resulting in kinase activation. Activated Trk receptors then phosphorylate a cascade of signaling molecules including the Ras/ERK, PI3K/Akt pathways and PLC-γ1. Activated Trk receptors also create internal docking sites for other signaling adaptor proteins to bind to5. Splice variants of TrkA, TrkB and TrkC have been observed. These splice isoforms are mainly affected in the tyrosine kinase domain of the receptor lying in the cytoplasm. Endocytosis is an important signaling trait of Trk receptors.
Following neurotrophin binding to the Trk receptor, the receptor complex is then internalized via endocytosis in order to terminate signaling. However, in the axonal compartment of neurons the internalization process of the neurotrophin complexed to the receptor is part of the signaling process and is important for activating transcription processes in the nucleus6,7.
