Overview
- Peptide HKLSEKLNPSVLRC, corresponding to amino acid residues 822-835 of mouse TRPC3 (Accession Q9QZC1). Intracellular, C-terminus.

 Western blot analysis of rat heart membranes:1. Anti-TRPC3 Antibody (#ACC-016), (1:200).
Western blot analysis of rat heart membranes:1. Anti-TRPC3 Antibody (#ACC-016), (1:200).
2. Anti-TRPC3 Antibody, preincubated with TRPC3 Blocking Peptide (#BLP-CC016).
- HEK-293 cells transfected with human TRPC3 (Kwan, H.Y. et al. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2625.).
 Expression of TRPC3 in rat pituitary glandImmunohistochemical staining of rat pituitary gland paraffin embedded sections using Anti-TRPC3 Antibody (#ACC-016), (1:100). TRPC3 is mainly expressed in the adenohypophysis (on left). Hematoxilin is used as the counterstain.
Expression of TRPC3 in rat pituitary glandImmunohistochemical staining of rat pituitary gland paraffin embedded sections using Anti-TRPC3 Antibody (#ACC-016), (1:100). TRPC3 is mainly expressed in the adenohypophysis (on left). Hematoxilin is used as the counterstain. Expression of TRPC3 in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-TRPC3 Antibody (#ACC-016) (A). Immunoreactivity appears in the molecular layer and in Purkinje cells (B).
Expression of TRPC3 in mouse cerebellumImmunohistochemical staining of mouse cerebellum using Anti-TRPC3 Antibody (#ACC-016) (A). Immunoreactivity appears in the molecular layer and in Purkinje cells (B). Expression of TRPC3 in rat cerebellumImmunohistochemical staining of TRPC3 in rat cerebellum using Anti-TRPC3 Antibody (#ACC-016). A. TRPC3 (green) appears in Purkinje cells (arrows) including both soma and dendrites and as well as in the molecular layer neuropil (asterisk). Staining of the same section with mouse anti-parvalbumin (red) reveals that TRPC3 is not expressed in molecular layer interneurons. B. Pre-incubating Anti-TRPC3 with the TRPC3 peptide antigen blocks staining. DAPI is used as the counterstain (blue).
Expression of TRPC3 in rat cerebellumImmunohistochemical staining of TRPC3 in rat cerebellum using Anti-TRPC3 Antibody (#ACC-016). A. TRPC3 (green) appears in Purkinje cells (arrows) including both soma and dendrites and as well as in the molecular layer neuropil (asterisk). Staining of the same section with mouse anti-parvalbumin (red) reveals that TRPC3 is not expressed in molecular layer interneurons. B. Pre-incubating Anti-TRPC3 with the TRPC3 peptide antigen blocks staining. DAPI is used as the counterstain (blue). Multiplex staining of TRPC6 and TRPC3 in rat cerebellumImmunohistochemical staining of rat cerebellum frozen section using Guinea pig Anti-TRPC6 Antibody (#ACC-017-GP) and rabbit Anti-TRPC3 Antibody (#ACC-016). A. TRPC6 staining (green) appears in molecular layer and in Purkinje cells. B. In the same section, staining of TRPC3 (red) appears as well in both molecular layer and Purkinje cells. C. Merge images of A and B indicates extensive co-localization. DAPI is used as the counterstain (blue).
Multiplex staining of TRPC6 and TRPC3 in rat cerebellumImmunohistochemical staining of rat cerebellum frozen section using Guinea pig Anti-TRPC6 Antibody (#ACC-017-GP) and rabbit Anti-TRPC3 Antibody (#ACC-016). A. TRPC6 staining (green) appears in molecular layer and in Purkinje cells. B. In the same section, staining of TRPC3 (red) appears as well in both molecular layer and Purkinje cells. C. Merge images of A and B indicates extensive co-localization. DAPI is used as the counterstain (blue).
 Expression of TRPC3 in rat C6 brain glioma cellsImmunocytochemical staining of Paraformaldehyde-fixed and permeabilized rat C6 brain glioma cells. A. Staining using Anti-TRPC3 Antibody (#ACC-016), (1:500) followed by goat anti-rabbit-AlexaFluor-488 secondary antibody. B. Nuclear staining using the cell-permeable dye Hoechst 33342. C. Merged image of panels A and B.
Expression of TRPC3 in rat C6 brain glioma cellsImmunocytochemical staining of Paraformaldehyde-fixed and permeabilized rat C6 brain glioma cells. A. Staining using Anti-TRPC3 Antibody (#ACC-016), (1:500) followed by goat anti-rabbit-AlexaFluor-488 secondary antibody. B. Nuclear staining using the cell-permeable dye Hoechst 33342. C. Merged image of panels A and B.
- Rat microglia cells (Mizoguchi, Y. et al. (2014) J. Biol. Chem. 289, 18549.).
- Moran, M.M. et al. (2004) Current Opin. Neurobiol. 14, 362.
- Clapham, D.E. et al. (2003) Pharmacol. Rev. 55, 591.
- Clapham, D.E. (2003) Nature 426, 517.
- Padinjat, R. and Andrews, S. (2004) J. Cell. Sci. 117, 5707.
- Huang, C.L. (2004) J. Am. Soc. Nephrol. 15, 1690.
- Vazquez, G. et al. (2003) J. Biol.Chem. 278, 21649.
- Venkatachalam, K. et al. (2003) J. Biol.Chem. 278, 29031.
The Transient Receptor Potential (TRP) superfamily is one of the largest ion channel families and consists of diverse groups of proteins. In mammals, about 28 genes encode the TRP ion channel subunits. The mammalian TRP superfamily comprises six subfamilies known as the TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPML (mucolipins), TRPP (polycystin) and the TRPA (ANKTM1) ion channels.1-4
The TRPC subfamily consists of seven proteins named TRPC1 to 7 which can be further divided into four subgroups based on their sequence homology and functional similarities:
1. TRPC1
2. TRPC4 and TRPC5
3. TRPC3, TRPC6, TRPC7
4. TRPC2.2,5
They are highly expressed in the central nervous system and to a lesser extent in peripheral tissues.
TRPC3, TRPC6 and TRPC7 form non-selective cationic channels that are activated by the stimulation of GPCRs.
Different modes of activation, DAG and SOC, were described for the TRPC3 channel by different investigators working with heterologous expression systems.6,7
Application key:
Species reactivity key:
Anti-TRPC3 Antibody (#ACC-016) is a highly specific antibody directed against an epitope of the mouse protein. The antibody can be used in western blot, immunoprecipitation, immunohistochemistry, immunocytochemistry, and indirect flow cytometry applications. It has been designed to recognize TRPC3 from mouse, rat, and human samples.
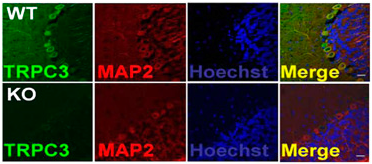
Knockout validation of Anti-TRPC3 Antibody in mouse cerebellumImmunohistochemical staining of mouse cerebellum sections using Anti-TRPC3 Antibody (#ACC-016). TRPC3 staining (green) is detected in neurons and partially overlaps with the neuronal marker MAP-2 (red). No staining for TRPC3 is observed in TRPC3-/- mice (lower panels).Adapted from Feng, S. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, 11011. with permission of the National Academy of Sciences of the United States of America.
Applications
Citations
 Expression of TRPC3 in rat cortex.Immunohistochemical staining of rat cortical brain slices using Anti-TRPC3 Antibody (#ACC-016). TRPC3 expression (green) is high in immature (P9) rat cortex and barely detectable in mature (P30) brain cortex. Its expression is high in cortical dysplasia (CD).
Expression of TRPC3 in rat cortex.Immunohistochemical staining of rat cortical brain slices using Anti-TRPC3 Antibody (#ACC-016). TRPC3 expression (green) is high in immature (P9) rat cortex and barely detectable in mature (P30) brain cortex. Its expression is high in cortical dysplasia (CD).
Adapted from Zhou, F.W. and Roger, S.N. (2014) with permission of the American Physiological Society. Upregulation of TRPC3 surface expression in rat microglia following BDNF stimulation.Immunocytochemical staining of rat microglia before (Control) and following BDNF stimulation (BDNF). A. Untreated cells were stained using Anti-TRPC3 Antibody (#ACC-016). BDNF-treated cells were stained using the same antibody. A significant increase in TRPC3 surface expression is observed following BDNF treatment. C. Same as in A. but including staining of CD45. D. Same as in B but including staining of CD45.
Upregulation of TRPC3 surface expression in rat microglia following BDNF stimulation.Immunocytochemical staining of rat microglia before (Control) and following BDNF stimulation (BDNF). A. Untreated cells were stained using Anti-TRPC3 Antibody (#ACC-016). BDNF-treated cells were stained using the same antibody. A significant increase in TRPC3 surface expression is observed following BDNF treatment. C. Same as in A. but including staining of CD45. D. Same as in B but including staining of CD45.
Adapted from Mizoguchi, Y. et al. (2014) with permission of The American Society for Biochemistry and Molecular Biology.
- Western blot analysis of mouse kidney lysate. Tested in TRPC3/6/7-/- mice.
Liu, B. et al. (2017) Am. J. Transl. Res. 9, 5619. - Western blot analysis of mouse brain lysate. Immunohistochemical staining of mouse cerebellum. Tested in Trpc3-/- mice.
Feng, S. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, 11011.
- Mouse kidney lysate. Also tested in TRPC3/6/7-/- mice.
Liu, B. et al. (2017) Am. J. Transl. Res. 9, 5619. - Human SH-SY5Y cell lysate and mouse brain lysate.
Sun, Y. et al. (2017) J. Neurosci. 37, 3364. - Rat neonatal ventricular cardiomyocytes (1:200).
Sabourin, J. et al. (2016) J. Biol. Chem. 291, 13394. - Human astroglioma U-87MG cell lysate.
Uemura, T. et al. (2016) Bipolar Dis. 19, 549. - Rat cerebral artery lysate.
Wang, Q. et al. (2016) Am. J. Physiol. 310, C1101. - Mouse brain lysate.
Neuner, S.M. et al. (2015) Behav. Brain Res. 281, 69. - Mouse airway smooth muscle cell lysate.
Song, T. et al. (2015) Am. J. Physiol. 309, L1455. - Rat insulinoma INS-1E cell lysate.
Sabourin, J. et al. (2015) J. Biol. Chem. 290, 30530. - Human coronary artery endothelial cells.
Ampem, P.T. et al. (2015) Vascul. Pharmacol. 76, 42. - Rat heart lysate.
Chen, M.S. et al. (2013) Cell. Physiol. Biochem. 32, 951. - Rat cortical astrocyte lysate (1:500).
Roedding, A.S. et al. (2013) Brain Res. 1517, 16. - Mouse brain lysate. Also tested in Trpc3-/- mice.
Feng, S. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, 11011. - Rat hippocampus lysate (1:2000).
Ryu, H.J. et al. (2013) Cell. Mol. Neurobiol. 33, 575.
- Rat neonatal ventricular cardiomyocytes.
Sabourin, J. et al. (2016) J. Biol. Chem. 291, 13394. - HEK-293 cells transfected with human TRPC3.
Kwan, H.Y. et al. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2625.
- Rat coronal brain sections.
Zhou, F.W. and Roger, S.N. (2014) J. Neurophysiol. 111, 1227. - Human bladder sections.
Kim, J.M. et al. (2013) Oncogenesis 2, e77. - Mouse cerebellum. Also tested in Trpc3-/- mice.
Feng, S. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, 11011. - Rat brain free-floating sections (1:200).
Ryu, H.J. et al. (2013) Cell. Mol. Neurobiol. 33, 575.
- Rat NRCMs (neonatal rat cardiomyocytes).
Sudi, S.B. et al. (2019) Sci. Rep. 9, 9785. - Rat primary cultured fetal and neonatal ventricular myocytes (1:100).
Jiang, Y. et al. (2014) Cell Tissue Res. 355, 201. - Rat microglia cells.
Mizoguchi, Y. et al. (2014) J. Biol. Chem. 289, 18549.
- Rat microglia cells.
Mizoguchi, Y. et al. (2014) J. Biol. Chem. 289, 18549.
- Alvarez-Miguel, I. et al. (2017) J. Physiol. 595, 1497.
- Chen, M. et al. (2017) J. Neural Transm. 124, 441.
- Jia, S. et al. (2017) Sci. Rep. 7, 5075.
- Liu, N. et al. (2017) Neurosci. Lett. 651, 1.
- Storck, H. et al. (2017) Oncotarget 8, 769.
- Wang, B. et al. (2017) J. Am. Heart Assoc. 6, e005812.
- Wang, X.C. et al. (2017) Sci. Rep. 7, 5895.
- Wang, Q. et al. (2016) Am. J. Physiol. 310, C1001.
- Kohda, K. et al. (2013) Proc. Natl. Acad. Sci. U.S.A. 110, E948.
- Lee, C.R. et al. (2013) J. Neurosci. 33, 1157.
- Li, W. et al. (2012) Proc. Natl. Acad. Sci. U.S.A. 109, 17087.
- Akita, T. and Okada, Y. (2011) J. Physiol. 589, 3909.
- Rosenberg, P. et al. (2004) Proc. Natl. Acad. Aci. U.S.A. 101, 9387.
- Castellano, L.E. et al. (2003) FEBS Lett. 541, 69.
- Trevino, C. L. et al. (2001) FEBS Lett. 509, 119.
