Overview
- Peptide (C)DQHVQDDTLHNVS, corresponding to amino acid residues 504-516 of human TRPC7 (Accession Q9HCX4). 2nd extracellular loop.
- Rat brain lysate, mouse brain membrane (1:200).
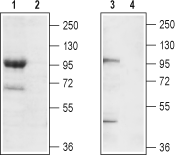 Western blot analysis of mouse brain membrane (lanes 1 and 2) and rat brain lysate (lanes 3 and 4):1,3. Anti-TRPC7 (extracellular) Antibody (#ACC-066), (1:200).
Western blot analysis of mouse brain membrane (lanes 1 and 2) and rat brain lysate (lanes 3 and 4):1,3. Anti-TRPC7 (extracellular) Antibody (#ACC-066), (1:200).
2,4. Anti-TRPC7 (extracellular) Antibody, preincubated with TRPC7 (extracellular) Blocking Peptide (#BLP-CC066).
- Rat PC12 cells (1:50).
Transient receptor potential (TRP) channels are relatively non-selective ion channels enabling the exchange of cations down their electrochemical gradient. This exchange enables the intracellular rise in Na+ and Ca2+ concentration and ultimately in the cell membrane depolarization, important for action potential propagation and muscle contraction1. They are activated by an extremely broad range of stimuli namely, temperature, voltage, pH, endocrine factors as well as signaling molecules2.
The TRP channel family is composed of 28 members divided in 7 subgroups: TRPV, TRPC, TRPM, TRPA, TRPN, TRPP and TRPML. All members of the TRP family form tetramers and could heterhomultimerize. They have 6 transmembrane (TM) domains, and a pore domain between the fifth (S5) and sixth (S6) transmembrane domains. In general, TRP channels enable the passage of either Na+ or Ca2+ ions with little or no preference. However, some channels do exhibit some selectivity. Also, TRP channels do not display the positive charges in the S4 voltage-sensing domain like most voltage sensitive channels, although they do display voltage dependency3. In addition, TRP channels have in the C-terminal intracellular region to the S6 domain a TRP domain comprising 25 amino acids that is more or less conserved among most TRP channels. Within the TRP domain, there is a TRP box composed of six amino acids, and TRP box 2 – a proline rich domain1,3. The TRP domain seems to be responsible for the binding of PIP2, a phospholipid important for the regulation of channel activity4.
The TRPC subfamily is further divided into the following: TRPC1/4/5, TRPC3/6/7 and TRPC21. Activation of phospholipase C (PLC) ultimately leads to the formation of diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3) via hydrolysis of PIP2. The increase in concentration of these intracellular second messengers leads to the activation of non-selective Ca2+ channels and an IP3-induced release of Ca2+ from intracellular stores5. The intracellular Ca2+ store depletion in turn activates Ca2+ specific channels to allow replenish intracellular Ca2+ levels. TRPCs are thought to be activated upon intracellular store Ca2+ depletion, and may function in concert along with the recently identified Orai channel5. The TRPC3/6/7 class produces similar currents upon activation.
TRPC7 is activated by a broad range of hormones and neurotrophins, many of which activate PLC dependent pathways, via G-protein coupled receptors (GPCRs) or receptor tyrosine kinases (RTKs). Its expression is has been detected in dorsal root ganglia (DRGs), heart, uterine myometrium, keratinocytes and leukemic cells5.
In myocardial cells, TRPC7 has recently been shown to promote apoptosis, thereby becoming a contributing factor in the development of heart disease. Indeed, there is a strong correlation between the high expression level of TRPC7 and apoptosis in hearts from Dahl salt-sensitive rats which have suffered from heart failure5,6.
Application key:
Species reactivity key:
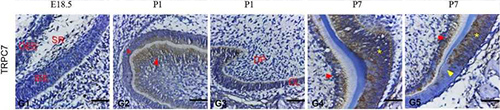
Expression of TRPC7 in rat tooth germ.Immunohistochemical staining of rat tooth germ sections using Anti-TRPC7 (extracellular) Antibody (#ACC-066). TRPC7 expression levels are high in ameloblasts and odontoblasts.Adapted from Yang, J. et al. (2017) Front. Physiol. 8, 456. with permission of Frontiers.
