Overview
- Peptide (C)RLDFYRLVQVDISFALK, corresponding to amino acid residues 212-228 of mouse TRPML2 (Accession Q8K595). Intracellular, N-terminus.
 Western blot analysis of human MCF-7 breast adenocarcinoma cell line (lanes 1 and 4), rat heart (lanes 2 and 5) and mouse heart (lanes 3 and 6) lysates:1-3. Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), (1:200).
Western blot analysis of human MCF-7 breast adenocarcinoma cell line (lanes 1 and 4), rat heart (lanes 2 and 5) and mouse heart (lanes 3 and 6) lysates:1-3. Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), (1:200).
4-6. Anti-TRPML2 (Mucolipin-2) Antibody, preincubated with TRPML2/Mucolipin-2 Blocking Peptide (#BLP-CC082).
 Expression of TRPML2 in rat heartImmunohistochemical staining of rat heart paraffin-embedded sections using Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), followed by AlexaFluor-555 secondary antibody. A. TRPML2 labeling appears specifically in the cardiac muscle fibers and in a lesser intensity in the endothelium. It does not appear in the smooth muscle fibers of blood vessels. B. Nuclear staining using DAPI as the counterstain. C. Merged images of A and B.
Expression of TRPML2 in rat heartImmunohistochemical staining of rat heart paraffin-embedded sections using Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), followed by AlexaFluor-555 secondary antibody. A. TRPML2 labeling appears specifically in the cardiac muscle fibers and in a lesser intensity in the endothelium. It does not appear in the smooth muscle fibers of blood vessels. B. Nuclear staining using DAPI as the counterstain. C. Merged images of A and B.
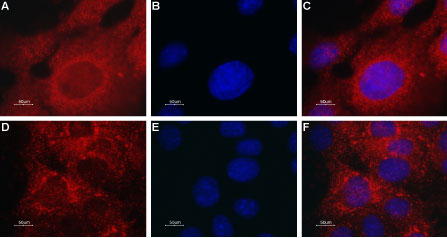 Expression of TRPML2 in mouse muscle myoblast (C2C12) cell lineImmunocytochemical staining of TRPML2 in C2C12 cells. A. Cells were stained with Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), (red), (1:50) followed by goat anti-rabbit-AlexaFluor-594 secondary antibody. B. DAPI is used as the counter staining (blue). C. Merged images of A and B.
Expression of TRPML2 in mouse muscle myoblast (C2C12) cell lineImmunocytochemical staining of TRPML2 in C2C12 cells. A. Cells were stained with Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082), (red), (1:50) followed by goat anti-rabbit-AlexaFluor-594 secondary antibody. B. DAPI is used as the counter staining (blue). C. Merged images of A and B.
- Cheng, X. et al. (2010) FEBS Lett. 584, 2013.
- Christensen, K.A. et al. (2002) J. Cell. Sci. 115, 599.
- Lange, I. et al. (2009) Sci. Signal. 2, ra23.
- Calcraft, P.J. et al. (2009) Nature 459, 596.
- Puertollano, R. and Kiselyov, K. (2009) Am. J. Physiol. 296, F1245.
- Sun, M. et al. (2000) Hum. Mol. Genet. 9, 2471.
- Samie, M.A. et al. (2009) Pflugers Arch. 459, 463.
- Cuajungco, M.P. et al. (2008) Pflugers Arch. 457, 463.
- Nagata, K. et al. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 353.
- Kim, H.J. et al. (2007) J. Biol. Chem. 282, 36138.
- Di Palma, F. et al. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14994.
The endolysosome system takes part in important cellular functions such as membrane trafficking, protein transport, autophagy and signal transduction1. Endosomes result from endocytosis of the plasma membrane and lysosomes (which are derived from late endosomes) conatin mainly hydrolytic enzymes and generally have a low internal pH1. Like the endoplasmic reticulum (ER), endolysosomes also store Ca2+ (luminal Ca2+ concentration: 0.5 mM)1,2, and similarly to Ca2+ release from the ER, Ca2+ from endolysosomes may also play an important role in various signaling events. To date such candidates include members of the TRP super-family of ion channels and the two-pore Ca2+ channels (TPCs)1,3,4.
TRPMLs, also termed mucolipins, are members of the TRP channels. In mammals, three TRPMLs are known to date (TRPML1-3 or MCOLN1-3). They are all localized to endolysosomes, although when over expressed in heterologous systems, TRPML3 is found on the plasma membrane1,5. These channels are Ca2+ permeable and display inward rectifying current properties1,5. Like all members of this family, TRPMLs have six transmembrane domains and intracellular N- and C-termini (relatively short tails compared to other members). They are characterized by an exceptionally large extracellular (luminal) loop between transmembrane domains 1 and 2, and N-glycosylation sites are present in the first extracellular (luminal) loop5.
In mammals, TRPML1 is expressed in a ubiquitous manner and shows highest expression in the brain, kidney, spleen, liver and heart1,6. TRPML2 and TRPML3 are less widely expressed. Interestingly, in mouse, two splice variants exist for TRPML2. The shorter variant is more broadly expressed and is dominant over the longer variant in the thymus, spleen and kidney1,7. TRPML3 is highly detected in the thymus, lung, kidney, spleen and eye1,7,8, some epithelial cells1,9 and brain10.
Pathologies related to these channels include type IV mucolipidosis, a neurodegenetative disease characterized by retardation and retinal degeneration caused by a loss of function mutation in the gene encoding TRPML1. In contrast, a gain of function mutation in TRPML3, in mice, causes deafness, and pigmentation defects11.
Application key:
Species reactivity key:
Anti-TRPML2 (Mucolipin-2) Antibody (#ACC-082) is a highly specific antibody directed against an epitope of the mouse protein. The antibody can be used in western blot, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize TRPML2 from human, rat, and mouse samples.
Applications
Citations
- Western blot and immunohistochemistry of human prostate cancer cell lines. Tested in cell lines treated with TRPML2 siRNA.
Yu, H. et al. (2021) Br. J. Cancer. 125, 1420.
- Mouse kidney sections (1:200).
Li, G. et al. (2019) Am. J. Physiol. 317, C481.
