Overview
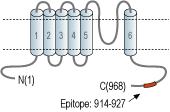
- Peptide (C)ERWESDDAASQISH, corresponding to amino acid residues 914-927 of human TRPP1 (Accession Q13563). Intracellular, C-terminus.
 Western blot analysis of rat kidney membrane proteins (lanes 1 and 3) and mouse kidney lysate (lanes 2 and 4):1,2. Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:200).
Western blot analysis of rat kidney membrane proteins (lanes 1 and 3) and mouse kidney lysate (lanes 2 and 4):1,2. Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:200).
3,4. Anti-TRPP1 (PKD2) Antibody, preincubated with TRPP1/PKD2 Blocking Peptide (#BLP-CC052).
 Expression of TRPP1 (PKD2) in rat kidneyImmunohistochemical staining of rat kidney paraffin embedded sections using Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:100), followed by goat anti-rabbit-Alexa-Fluor-594 secondary antibody. A. TRPP1 staining followed by goat anti-rabbit-Alexa-Fluor-594 secondary antibody (red) appears in the renal cortex, mainly in the distal tubules. B. DAB staining of TRPP1 (brown) also shows that the renal corpuscle and the blood vessels are not stained at all. (PT = proximal tubule, DT = distal tubule).
Expression of TRPP1 (PKD2) in rat kidneyImmunohistochemical staining of rat kidney paraffin embedded sections using Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:100), followed by goat anti-rabbit-Alexa-Fluor-594 secondary antibody. A. TRPP1 staining followed by goat anti-rabbit-Alexa-Fluor-594 secondary antibody (red) appears in the renal cortex, mainly in the distal tubules. B. DAB staining of TRPP1 (brown) also shows that the renal corpuscle and the blood vessels are not stained at all. (PT = proximal tubule, DT = distal tubule).
 Expression of TRPP1 (PKD2) in C2C12 cell lineImmunocytochemical staining of TRPP1 (PKD2) in mouse paraformaldehyde-fixed and permeabilized muscle myoblasts (C2C12) cell line. A. Cells were stained with Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:50-1:100) followed by goat anti-rabbit-AlexaFluor-594 secondary antibody (red). B. Nuclear staining using DAPI as the counterstain (blue). C. Merged images of panels A and B.
Expression of TRPP1 (PKD2) in C2C12 cell lineImmunocytochemical staining of TRPP1 (PKD2) in mouse paraformaldehyde-fixed and permeabilized muscle myoblasts (C2C12) cell line. A. Cells were stained with Anti-TRPP1 (PKD2) Antibody (#ACC-052), (1:50-1:100) followed by goat anti-rabbit-AlexaFluor-594 secondary antibody (red). B. Nuclear staining using DAPI as the counterstain (blue). C. Merged images of panels A and B.
- Ramsey, I.S. et al. (2006) Annu. Rev. Physiol. 68, 619.
- Pedersen, S.F. et al. (2005) Cell Calcium 38, 233.
- Montell, C. (2005) Sci. STKE 2005, re3.
- Hilgemann, D.W. et al. (2001) Sci. STKE 2001, re19.
- Tsiokas, L. (2009) Am. J. Renal. Physiol. 297, F1.
- Owsianik, G. et al. (2006) Annu. Rev. Physiol. 68, 685.
- Koulen, P. et al. (2002) Nat. Cell Biol. 4, 191.
- Giamarchi, A. et al. (2006) EMBO Rep. 7, 787.
- Wilson, P.D. (2001) J. Am. Soc. Nephrol. 12, 834.
- Venkatachalam, K. and Montell, C. (2007) Annu. Rev. Biochem. 76, 387.
- Hanaoka, K. et al. (2000) Nature 408, 990.
- Mochizuki, T. et al. (1996) Science 272, 1339.
Transient receptor potential (TRP) channels are relatively non-selective ion channels that enable the flow of cations down their electrochemical gradient. This enables the increase in intracellular Na+ and Ca2+ concentrations and ultimately in the cell membrane depolarization, which is important for action potential propagation and muscle contraction1. They are activated by a broad range of stimuli, namely temperature, voltage, pH, endocrine factors, as well as signaling molecules2.
The TRP channel family is composed of 28 members divided into 7 subgroups: TRPV, TRPC, TRPM, TRPA, TRPN, TRPP and TRPML. All members of the TRP family have 6 transmembrane (TM) domains, with the pore between the fifth (S5) and sixth (S6) TM domains. In general, TRP channels enable the passage of either Na+ or Ca2+ ions with little to no preference.
However, some channels do exhibit some selectivity. Also, TRP channels do not display the positive charges in the S4 voltage-sensing domain like most voltage-sensitive channels, although they do display voltage-dependency3. In addition, the C-terminal, located intracellularly, contains a TRP domain comprising 25 amino acids that are highly conserved between TRP channels. Within the TRP domain, there is a TRP box composed of six amino acids, and TRP box 2 – a proline rich domain1,3. The TRP domain seems to be responsible for the binding of PIP2, a phospholipid important for the regulation of channel activity4.
TRPP1 (polycystin-2, PC2, PKD2) belongs to the TRPP subfamily along with TRPP3 and TRPP5 proteins, and forms non-selective cation channels with different permeability to various divalent cations5,6. The cellular localization of TRPP1 has been and still is the subject of a lasting debate. In many cell-types, TRPP1 is retained in the endoplasmic reticulum (ER) where it most likely functions as a Ca2+ release channel7,8, and with the help of cofactors, TRPP2 reaches the plasma membrane and the cilia8. TRPP1 expression is widespread and is best characterized for its expression in the kidney where it is developmentally regulated9,10. In the kidney, it associates with PKD1 (TRPP2) an eleven TM-spanning protein (which does not belong to the TRP superfamily) to form functional channels11. In addition, TRPP1 is identified as one of the genes responsible for autosomal dominant polycystic kidney disease (ADPKD)5,12.
Application key:
Species reactivity key:
Anti-TRPP1 (PKD2) Antibody (#ACC-052) is a highly specific antibody directed against an epitope of human Polycystin-2. The antibody can be used in western blot, immunohistochemistry and immunocytochemistry applications. It has been designed to recognize TRPP1 from human, rat, and mouse samples.
