Overview
- Peptide CDGHQQGYAPKWRAEDAPL, corresponding to amino acid residues 853-871 of rat TRPV4 (Accession Q9ERZ8). Intracellular, C-terminus.

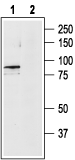 Western blot analysis of rat brain lysates:1. Anti-TRPV4 Antibody (#ACC-034), (1:200).
Western blot analysis of rat brain lysates:1. Anti-TRPV4 Antibody (#ACC-034), (1:200).
2. Anti-TRPV4 Antibody, preincubated with TRPV4 Blocking Peptide (#BLP-CC034). Western blot analysis of ND7/23 Mouse neuroblastoma /rat dorsal root ganglion neuron hybrid cell line lysate:1. Anti-TRPV4 Antibody (#ACC-034), (1:200).
Western blot analysis of ND7/23 Mouse neuroblastoma /rat dorsal root ganglion neuron hybrid cell line lysate:1. Anti-TRPV4 Antibody (#ACC-034), (1:200).
2. Anti-TRPV4 Antibody, preincubated with TRPV4 Blocking Peptide (#BLP-CC034).
- Rat and mouse brain lysates (10 μg Ab/0.500 mg whole-protein) (Benfenati, V. et al. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 2563).
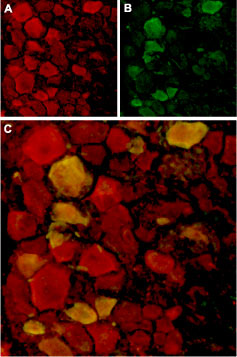 Expression of TRPV4 in rat DRGImmunohistochemical staining of rat dorsal root ganglion (DRG) frozen sections using Anti-TRPV4 Antibody (#ACC-034). A. TRPV4 (red) in DRG neurons. B. Staining with mouse anti-Parvalbumin (green) in the same DRG section. C. Confocal merge of TRPV4 and Parvalbumin demonstrates colocalization.
Expression of TRPV4 in rat DRGImmunohistochemical staining of rat dorsal root ganglion (DRG) frozen sections using Anti-TRPV4 Antibody (#ACC-034). A. TRPV4 (red) in DRG neurons. B. Staining with mouse anti-Parvalbumin (green) in the same DRG section. C. Confocal merge of TRPV4 and Parvalbumin demonstrates colocalization.
 Expression of TRPV4 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture.
Expression of TRPV4 in rat DRG primary cultureImmunocytochemical staining of paraformaldehyde-fixed and permeabilized rat dorsal root ganglion (DRG) primary culture.
A, D. Staining using Anti-TRPV4 Antibody (#ACC-034), (1:500), followed by goat anti-rabbit-AlexaFluor-555 secondary antibody.
B, E. Nuclear staining of cells using the cell-permeable dye Hoechst 33342.
C. Merged image of panels A and B.
F. Merged image of panels D and E.
- Human corneal epithelial cells (HCEC) (1:200) (Pan, Z. et al. (2008) Cell Calcium 44, 374.).
- The control antigen is not suitable for this application.
- Montell, C. et al. (2002) Mol. Cell. 9, 229.
- Clapham, D.E. (2003) Nature 426, 517.
- Moran, M.M. et al. (2004) Curr.Opin.Neurobiol. 14, 362.
- Clapham, D.E. et al. (2003) Pharmacol. Rev. 55, 591.
- Gunthorpe, M.J.et al. (2002) Trends. Pharmacol. Sci. 23, 183.
- Peng, J.B. et al. (2003) J. Physiol. 551.3, 729.
TRP channels are a large family (about 28 genes) of plasma membrane, non-selective cationic channels that are either specifically or ubiquitously expressed in excitable and non-excitable cells.1 According to IUPHAR the TRP family comprises of three main subfamilies on the basis of sequence homology; TRPC, TRPM and TRPV (to date, three extra subfamilies are considered to belong to the TRP family; the TRPA, TRPML, and TRPP).1-4 The TRPV subfamily consists of six members, TRPV1-6.5
TRPV4 (also named OTRPC4) is activated under hypotonic conditions and serves as an osmoreceptor. TRPV4 is expressed in brain, liver, kidney, heart, testis and salivary gland.6
Application key:
Species reactivity key:
Anti-TRPV4 Antibody (#ACC-034) is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot, immunoprecipitation, indirect flow cytometry, immunohistochemistry, and immunocytochemistry applications. It has been designed to recognize TRPV4 from human, rat, and mouse samples.
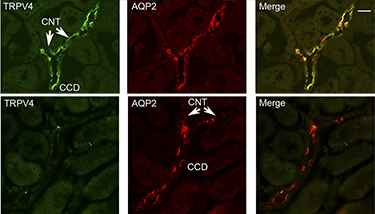
Knockout validation of Anti-TRPV4 Antibody in mouse kidney.Immunohistochemical staining of mouse kidney sections using Anti-TRPV4 Antibody (#ACC-034) and Anti-Aquaporin 2-ATTO Fluor-550 Antibody (#AQP-002-AO). TRPV4 staining (green, upper panels) is detected in both the connecting tubules (CNT) and the collecting ducts (CCD). AQP2 immunostaining (red) is also observed in the CCD and CNT. TRPV4 staining is not observed in the sections from TRPV4-/- mice. Adapted from Berrout, J. et al. (2012) J. Biol. Chem. 287, 8782. with permission of American Society for Biochemistry and Molecular Biology.
Applications
Citations
 Expression of TRPV4 in rat perihematomal area following ICH.Immunohistochemical staining of rat brain sections 24 hours after intracerebral hemorrhage (ICH) induction using Anti-TRPV4 Antibody (#ACC-034). At 24 hours post-ICH, TRPV4 immunostaining (red) was detected on neurovascular structures, perivascular astrocytes and endothelial cells in the perihematomal area. TRPV4 staining coincided with that of GFAP (lower panels), an astrocyte marker and von Willebrand factor (vWF) (upper panels), a marker of BBB. Nuclei were stained using DAPI.
Expression of TRPV4 in rat perihematomal area following ICH.Immunohistochemical staining of rat brain sections 24 hours after intracerebral hemorrhage (ICH) induction using Anti-TRPV4 Antibody (#ACC-034). At 24 hours post-ICH, TRPV4 immunostaining (red) was detected on neurovascular structures, perivascular astrocytes and endothelial cells in the perihematomal area. TRPV4 staining coincided with that of GFAP (lower panels), an astrocyte marker and von Willebrand factor (vWF) (upper panels), a marker of BBB. Nuclei were stained using DAPI.
Adapted from Zhao, H. et al. (2018) Front. Mol. Neurosci. 11, 97. with permission of Frontiers. Multiplex staining of TRPV4 and KCa3.1 in human BSM cells.Immunocytochemical staining of human bronchial smooth muscle (HBSM) cells using Mouse Anti-KCNN4 (KCa3.1, SK4) (extracellular) Antibody (#ALM-051), (green) and Anti-TRPV4 Antibody (#ACC-034), (red).
Multiplex staining of TRPV4 and KCa3.1 in human BSM cells.Immunocytochemical staining of human bronchial smooth muscle (HBSM) cells using Mouse Anti-KCNN4 (KCa3.1, SK4) (extracellular) Antibody (#ALM-051), (green) and Anti-TRPV4 Antibody (#ACC-034), (red).
Adapted from Yu, Z. et al. (2017) Front. Pharmacol. 8, 559. with permission of Frontiers. Downregulation of TRPV4 in rat retinal microvascular endothelium.Immunohistochemical staining of rat retinal sections using Anti-TRPV4 Antibody (#ACC-034). A. TRPV4 expression (green) is mostly detected in the endothelium of retinal microvessels. B. Specificity of the antibody is depicted by showing loss of TRPV4 staining when pre-incubating with the control peptide antigen. C. Significant decrease in TRPV4 expression is observed in retina section from diabetic animals.
Downregulation of TRPV4 in rat retinal microvascular endothelium.Immunohistochemical staining of rat retinal sections using Anti-TRPV4 Antibody (#ACC-034). A. TRPV4 expression (green) is mostly detected in the endothelium of retinal microvessels. B. Specificity of the antibody is depicted by showing loss of TRPV4 staining when pre-incubating with the control peptide antigen. C. Significant decrease in TRPV4 expression is observed in retina section from diabetic animals.
Adapted from Monaghan, K. et al. (2015) with kind permission of Curtis, T.M., Centre for Experimental Medicine, Queen’s University of Belfast.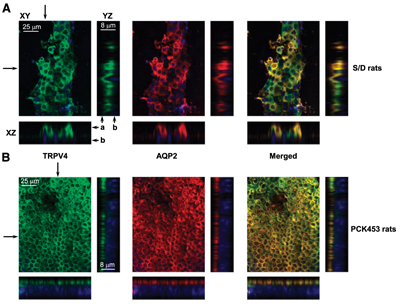 Multiplex staining of Aquaporin 2 and TRPV4 in rat kidney.Immunohistochemical staining of rat kidney sections using Anti-TRPV4 Antibody (#ACC-034) and Anti-Aquaporin 2-ATTO Fluor-550 Antibody (AQP-002-AO). Shown are Representative confocal plane micrographs (axes are shown) and corresponding cross-sections (pointed by arrows) showing three-dimensional stacks of TRPV4 (green), AQP2 localization (red), and the combined image localization (yellow). A. Wild-type Sprague-Dawley (S/D) rat. B. Cyst monolayer from a PCK453 rat. DAPI staining is shown in blue.
Multiplex staining of Aquaporin 2 and TRPV4 in rat kidney.Immunohistochemical staining of rat kidney sections using Anti-TRPV4 Antibody (#ACC-034) and Anti-Aquaporin 2-ATTO Fluor-550 Antibody (AQP-002-AO). Shown are Representative confocal plane micrographs (axes are shown) and corresponding cross-sections (pointed by arrows) showing three-dimensional stacks of TRPV4 (green), AQP2 localization (red), and the combined image localization (yellow). A. Wild-type Sprague-Dawley (S/D) rat. B. Cyst monolayer from a PCK453 rat. DAPI staining is shown in blue.
Adapted from Zaika, O. et al. (2013) J. Am. Soc. Nephrol. 24, 604. with permission of the American Society of Nephrology. PKA activation causes TRPV4 subcellular translocation.Immunocytochemical staining of TRPV4 expression in split-opened nephrons using Anti-TRPV4 Antibody (#ACC-034). A. control conditions. B. Activation of PKA with forskolin leads to TRPV4 translocation to the apical plasma membrane. C. Activation of PKC with PMA has no effect. D. pre-treatment with PKA specific inhibitor, abolishes TRPV4 translocation.
PKA activation causes TRPV4 subcellular translocation.Immunocytochemical staining of TRPV4 expression in split-opened nephrons using Anti-TRPV4 Antibody (#ACC-034). A. control conditions. B. Activation of PKA with forskolin leads to TRPV4 translocation to the apical plasma membrane. C. Activation of PKC with PMA has no effect. D. pre-treatment with PKA specific inhibitor, abolishes TRPV4 translocation.
Adapted from Mamenko, M. et al. (2013) with permission of American Society for Biochemistry and Molecular Biology.
- Western blot and immunohistochemistry of TRPV4 in mouse mesenteric artery. Tested in TRPV4 knockout (TRPV4−/−) mice.
Zhu, Y. et al. (2021) Cardiovasc. Res. 117, 2450. - Immunohistochemistry of TRPV4 in mouse carotid artery. Tested in endothelium-specific TRPV4 knockout mice.
McFarland, S.J. et al. (2020) Int. J. Mol. Sci. 21, 2179. - Immunocytochemistry of TRPV4 in human annulus fibrosus (AF) cells. Tested in cells with TRPV4 knocked out using CRISPR-Cas9.
Cambria, E. et al. (2020) Cells. 9, 1736. - Western blot and immunocytochemistry of TRPV4 in normal mouse primary epidermal keratinocytes (NMEKs). Tested in TRPV4 knockout (TRPV4−/−) mice.
Sharma, S. et al. (2019) J. Cell. Mol. Med. 23, 761. - Immunohistochemical staining of mouse kidney sections. Tested in TRPV4-/- mice.
Berrout, J. et al. (2012) J. Biol. Chem. 287, 8782.
- Human hCMEC/D3 BBB and HUVEC cell lysates (1:200).
Kumar, H. et al. (2020) J. Neurosci. 40, 1943. - Mouse pancreas islet lysate (1:400).
Sawatani, T. et al. (2019) Am. J. Physiol. 316, C434. - Rat microvascular endothelial cells.
Suresh, K. et al. (2018) Am. J. Physiol. 314, L893. - Mouse heart lysates.
Dong, Q. et al. (2017) Sci. Rep. 7, 42678. - Rat cerebral arterial myocyte lysates.
Gebremedhin, D. et al. (2017) PLoS ONE 12, e0176796. - Rat neonatal ventricle myocyte lysates.
Wu, Q.F. et al. (2017) Cell Death Dis. 8, e2828. - Mouse CCDcl1 cell lysate (1:1000).
Li, Y. et al. (2016) PLoS ONE 11, e0155006. - Mouse DRG lysate (1:5000).
Kim, S. et al. (2016) Sci. Signal. 9, Ra71. - Mouse mesenteric arteries lysate.
Yap, F.C. et al. (2016) Am. J. Physiol. 310, H1151. - Mouse bone marrow-derived macrophage lysate.
Scheraga, R.G. et al. (2016) J. Immunol. 196, 428. - Human microvascular endothelial cell (HMEC-1) lysate (1:2000).
Goedicke-Fritz, S. et al. (2015) Eur. J. Cell Biol. 94, 391. - Mouse microvascular endothelial cell lysate (1:500).
Suresh, K. et al. (2015) Am. J. Physiol. 309, L1467. - Rat myometrial protein lysate (1:200).
Ying, L. et al. (2015) Sci. Transl. Med. 7, 319ra204. - Mouse adipose tissue lysate.
Castellani, L. et al. (2014) J. Appl. Physiol. 116, 1272. - Human preadipocytes.
Che, H. et al. (2014) Pflugers Arch. 466, 947. - RPMVEC cell lysate.
Parker, J.C. et al. (2013) Physiol. Rep. 1, e00121.
- Rat cortical collecting duct cells.
Pizzoni, A. et al. (2018) J. Cell Biochem. 119, 4120. - Mouse DRG lysates.
Kim, S. et al. (2016) Sci. Signal. 9, Ra71. - Rat and mouse brain lysates (10 µg/500 mg lysate).
Benfenati, V. et al. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 2563.
- Rat and mouse spinal cord sections (1:500).
Kumar, H. et al. (2020) J. Neurosci. 40, 1943. - Mouse pancreas sections.
Sawatani, T. et al. (2019) Am. J. Physiol. 316, C434. - Rat brain sections.
Zhao, H. et al. (2018) Front. Mol. Neurosci. 11, 97. - Mouse heart sections.
Dong, Q. et al. (2017) Sci. Rep. 7, 42678. - Rat cerebral arterial segment sections.
Gebremedhin, D. et al. (2017) PLoS ONE 12, e0176796. - Mouse cornea sections (1:200).
Okada, Y. et al. (2016) PLoS ONE 11, e0167200. - Mouse kidney sections (1:200).
Li, Y. et al. (2016) PLoS ONE 11, e0155006. - Mouse mesenteric arteries sections.
Yap, F.C. et al. (2016) Am. J. Physiol. 310, H1151. - Rat trigeminal sections (1:100).
Meng, Q. et al. (2015) Am. J. Physiol. 309, C1. - Rat retinal sections.
Monaghan, K. et al. (2015) PLoS ONE 10, e0128359. - Rat kidney sections (1:500).
Zaika, O. et al. (2013) J. Am. Soc. Nephrol. 24, 604. - Mouse kidney sections. Also tested in TRPV4-/- mice.
Berrout, J. et al. (2012) J. Biol. Chem. 287, 8782.
- Human hCMEC/D3 BBB cells.
Kumar, H. et al. (2020) J. Neurosci. 40, 1943. - Rat cerebral arterial myocytes.
Gebremedhin, D. et al. (2017) PLoS ONE 12, e0176796. - Human bronchial smooth muscle cells.
Yu, Z. et al. (2017) Front. Pharmacol. 8, 559. - Mouse CCDcl1 cells (1:200).
Li, Y. et al. (2016) PLoS ONE 11, e0155006. - Rat myometrial smooth muscle cells (1:200).
Ying, L. et al. (2015) Sci. Transl. Med. 7, 319ra204. - Rat pulmonary microvascular endothelial cells (RPMVEC) (1:200).
Parker, J.C. et al. (2013) Physiol. Rep. 1, e00121. - Mouse distal nephrons (1:1000).
Mamenko, M. et al. (2013) J. Biol. Chem. 288, 20306.
- Human hCMEC/D3 BBB cells.
Kumar, H. et al. (2020) J. Neurosci. 40, 1943. - Rat and mouse spinal cord sections (1:500).
Kumar, H. et al. (2020) J. Neurosci. 40, 1943. - Mouse pancreas sections.
Sawatani, T. et al. (2019) Am. J. Physiol. 316, C434.
- Human corneal epithelial cells (HCEC) (1:200).
Pan, Z. et al. (2008) Cell Calcium 44, 374.
- Wen, L. et al. (2018) BMC Ophthalmology 18, 38.
- Zhao, H. et al. (2018) Front. Mol. Neurosci. 11, 97.
- Jo, A.O. et al. (2016) Proc. Natl. Acad. Sci. U.S.A. 113, 3885.
- Alexander, R. et al. (2013) Br. J. Pharmacol. 168, 761.
- Fawcett, K.A. et al. (2012) J. Neurol. Neurosurg. Psychiatry 83, 1204.
- Ye, L. et al. (2012) Cell 151, 96.
