Overview
- Peptide (C)KHEKNSTEIQT(A)R, corresponding to amino acid residues 52-64 of rat VMAT2 (Accession Q01827). 1st luminal loop.
- Rat brain sections (1:60).
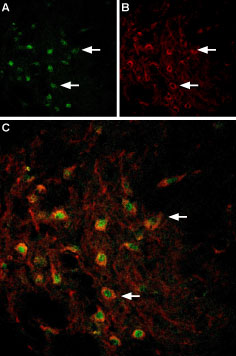 Multiplex staining of VMAT2 and TRPC3 in rat brainImmunohistochemical staining of perfusion-fixed frozen rat substantia nigra sections using Anti-VMAT2-ATTO Fluor-488 Antibody (#AMT-006-AG), (1:60) and Anti-TRPC3-ATTO Fluor-594 Antibody (#ACC-016-AR), (1:60). A. VMAT2 staining (green). B. The same section stained for TRPC3 (red). C. Merged image demonstrates the ubiquitous colocalization of VMAT2 and TRPC3 in cells of the substantia nigra pars compacta. Arrows point at examples of co-expression.
Multiplex staining of VMAT2 and TRPC3 in rat brainImmunohistochemical staining of perfusion-fixed frozen rat substantia nigra sections using Anti-VMAT2-ATTO Fluor-488 Antibody (#AMT-006-AG), (1:60) and Anti-TRPC3-ATTO Fluor-594 Antibody (#ACC-016-AR), (1:60). A. VMAT2 staining (green). B. The same section stained for TRPC3 (red). C. Merged image demonstrates the ubiquitous colocalization of VMAT2 and TRPC3 in cells of the substantia nigra pars compacta. Arrows point at examples of co-expression.
Vesicular monoamine transporters (VMATs) are essential for proper monoaminergic neurotransmission, which requires the sequestration of the transmitter into synaptic vesicles by VMAT for subsequent Ca2+-stimulated exocytotic release. VMATs contain 12 transmembrane spanning domains, with cytosolic C- and N-terminals and large glycosylated intravesicular loops. In mammals, there are two VMAT isoforms: VMAT1 (SLC18A1) and VMAT2 (SLC18A2)1.
VMATs primarily transport monoamines (dopamine, serotonin, norepinephrine and histamine), but also sequester toxicants into vesicles, shunting them away from cytosolic sites of action. In the central nervous system, VMAT2 is the only transporter that moves cytoplasmic dopamine (DA) into synaptic vesicles for storage and subsequent exocytotic release2.
VMAT2 is expressed in monoaminergic neurons of the central nervous system (CNS) and sympathetic postganglionic neurons. VMAT2 is widely expressed during embryonic development3. Homozygous knock-out of VMAT2 in mice is lethal and heterozygous knock-out of VMAT2 in mice shows a clear gene dosage effect, with half the amount of dopamine, norepinephrine, and serotonin content in brain4. In addition VMAT2 plays a role in neuroprotection and molecules that interact with VMAT2 may have value as treatments for diseases such as Parkinson’s disease5.
