Overview
- Zhang, C. et al. (2017) Proc. Natl. Acad. Sci. U.S.A. 114, e2524.
BomoTx belongs to a group of secreted phospholipase A2 (sPLA2)-like protein toxins originally isolated from the Brazilian lancehead pit viper (Bothrops moojeni). This toxin contains a conserved PLA2 fold but lacks enzymatic activity. BomoTx is mostly related to Lys49 myotoxins found in snake species within the Crotalinae subfamily1.
BomoTx functions by activating a subpopulation of somatosensory neurons that contribute to pain sensation. BomoTx excites these neurons by stimulating the release of cellular ATP through a mechanism involving pannexin hemichannels resulting in activation of P2X2 and P2X3 purinergic receptors. Injection of BomoTx to mouse hind paw causes non-neurogenic inflammatory pain, thermal hyperalgesia, and mechanical allodynia.
P2X receptors are cationic trimeric channel complexes that function as ATP-gated calcium-permeable channels and are attractive therapeutic targets. This family contains seven receptor subtypes: P2X1–P2X72.
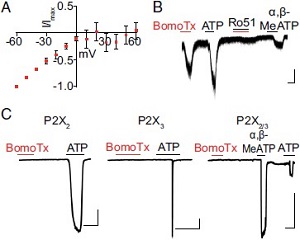
BomoTx-induced currents are mediated by ATP.A. Representative current–voltage relationship of BomoTx-evoked conductance from transiently activated TG neurons demonstrates inwardly rectified currents that reverse at 0 mV. B. Representative whole-cell voltage-clamp experiments on TG neurons showing that Ro51 (a P2X3 receptor antagonist) blocks BomoTx-evoked current. C. BomoTx does not directly activate HEK cells expressing rat P2X2, P2X3, or P2X2/3 channels.Adapted from Zhang, C. et al. (2017) Proc. Natl. Acad. Sci. U.S.A. 114, e2524. with permission of the National Academy of Sciences.

