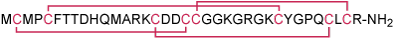Overview
- DeBin, J.A. et al. (1993) Am. J. Physiol. 264, C361.
- Soroceanu, L. et al. (1999) J. Neurosci. 19, 5942.
- DeBin, J.A. and Strichartz, G.R. (1991) Toxicon 29, 1403.
- DeBin, J.A. et al. (1993) Am. J. Physiol. 264, C361.
- Soroceanu, L. et al. (1999) J. Neurosci. 19, 5942.
- Ullrich, N. et al. (1998) Neuroscience 83, 1161.
- Ullrich, N. et al. (1996) Neuroreport 7, 1020.
- Soroceanu, L. et al. (1998) Cancer Res. 58, 4871.
- Lyons, S.A. et al. (2002) Glia 39, 162.
- Deshane, J. et al. (2003) J. Biol. Chem. 278, 4135.
- McFerrin, M.B. and Sontheimer, H. (2006) Neuron Glia Biol. 2, 39.
Native chlorotoxin (CTX) is a 36-amino acid peptide, originally isolated from the venom of the giant yellow Israeli scorpion Leiurus quinquestriatus hebraeus. Its primary amino acid sequence shows considerable homology to a class of short insectotoxins.1
Initially, CTX was demonstrated as an irreversible inhibitor of small conductance Cl- channels in colonic epithelial cells and Cl- fluxes across glioma cell membranes.2,3 Inhibition of Cl- channels with CTX prevents cell volume changes, and in turn, inhibits tumor cell invasion and migration.4-6
Immunohistochemical studies show that CTX specifically and selectively binds to glioma cell lines and to tissue biopsies from patients with various malignant gliomas and other embryonic related tumors of neuroectodermal origin but not to normal brain tissue.
These findings have lead to clinical evaluation for the therapeutic and diagnostic use of CTX, a synthetic derivate, in patients with malignant glioma. This derivative of CTX has been shown to selectively label human gliomas in vivo and in vitro and demonstrated all of the known physical and biological activities of the naturally occurring CTX.7,8
Further studies on the interaction of CTX with Cl- channels suggest that these effects are indirect; affinity purification with a recombinant CTX identified the matrix metalloproteinase-2 (MMP2) as a CTX binder in glioma cells. The actual receptor for CTX appears to be a protein complex that contains MMP2 and Cl- channel 3 (ClC-3). Binding of CTX induces endocytosis of this complex and hence the ClC-3 channels. This finding might explain the irreversible action of CTX and its relatively slow time course of Cl- channel blockage.9,10
Chlorotoxin (#STC-460) is a highly pure, synthetic, and biologically active peptide toxin.