Overview
Cat #:
STG-200
Alternative Name GxTx-1E, κ-Theraphotoxin-Pg1a, κ-TRTX-Pg1a, Guangxitoxin 1E
Lyophilized Powder yes
Origin Synthetic peptide
MW: 3948.7 Da
Purity: >98% (HPLC)
Effective concentration 1-100 nM.
Sequence EGECGGFWWKCGSGKPACCPKYVCSPKWGLCNFPMP.
Modifications Presumed disulfide bonds between Cys4-Cys19, Cys11-Cys24 and Cys18-Cys31 (disulfide bonds undetermined).
Molecular formula C178H248N44O45S7.
Activity Guangxitoxin-1E is a gating modifier of KV2.1 (KCNB1, IC50 of 1 nM), KV2.2 (KCNB2, IC50 of 3 nM) and KV4.3 (KCND3, IC50 of 10-20 fold higher concentration) channels. In pancreatic β cells it enhances glucose-stimulated insulin secretion by broadening the cell action potential and enhancing calcium oscillations1,2. The physiological modulation of KV2.1 channels during glucose-induced insulin secretion is MgATP-mediated3.
References-Activity
- Herrington, J. et al. (2006) Diabetes 55, 1034.
- Herrington, J. (2007) Toxicon 49, 231.
- Yoshida, M. et al. (2010) Biochem. Biophys. Res. Commun. 396, 304.
Accession number P84835
Shipping and storage Shipped at room temperature. Product as supplied can be stored intact at room temperature for several weeks. For longer periods, it should be stored at -20°C.
Solubility Any aqueous buffer. Centrifuge all product preparations before use (10000 x g 5 min).
Storage of solutions Up to two weeks at 4°C or three months at -20°C.
Our bioassay
 Alomone Labs Guangxitoxin-1E inhibits KV2.1 channel currents expressed in Xenopus oocytes.Currents were elicited by application of voltage ramp from a holding potential of -80 mV to 60 mV in 100 msec, delivered every 10 seconds. A. Time course of channel activity (current amplitude at +40 mV), before (black) and during (green) application of 100 nM Guangxitoxin-1E (#STG-200). B. Top, illustration of the voltage ramp protocol. Bottom, example of superimposed current traces before (black) and during (green) application of 100 nM Guangxitoxin-1E, taken from the experiment in A.
Alomone Labs Guangxitoxin-1E inhibits KV2.1 channel currents expressed in Xenopus oocytes.Currents were elicited by application of voltage ramp from a holding potential of -80 mV to 60 mV in 100 msec, delivered every 10 seconds. A. Time course of channel activity (current amplitude at +40 mV), before (black) and during (green) application of 100 nM Guangxitoxin-1E (#STG-200). B. Top, illustration of the voltage ramp protocol. Bottom, example of superimposed current traces before (black) and during (green) application of 100 nM Guangxitoxin-1E, taken from the experiment in A.
References - Scientific background
- Herrington, J. et al. (2006) Diabetes 55, 1034.
- Herrington, J. (2007) Toxicon 49, 231.
- Yoshida, M. et al. (2010) Biochem. Biophys. Res. Commun. 396, 304.
- Yoshida M. et al. (2009) FEBS Lett. 583, 2225.
Scientific background Guangxitoxin-1E is a 36 amino acid peptidyl toxin isolated from the Chilobrachys jingzhao (Chinese earth tiger) tarantula venom and belongs to the huwentoxin-1 family. Guangxitoxin-1E is a gating modifier of KV2.1 (KCNB1, IC50 of 1 nM), KV2.2 (KCNB2, IC50 of 3 nM) and KV4.3 (KCND3, IC50 of 50 nM) channels1. In pancreatic β cells, it enhances glucose-stimulated insulin secretion by broadening the cell action potential and enhancing calcium oscillations1,2. The physiological modulation of KV2.1 channels during glucose-induced insulin secretion is MgATP-mediated3,4. No significant effect was found on KV1.2 (KCNA2), KV1.3 (KCNA3), KV1.5 (KCNA5), KV3.2 (KCNC2), CaV1.2 (CACNA1C), CaV2.2 (CACNA1B), NaV1.5 (SCN5A), NaV1.7 (SCN9A) or NaV1.8 (SCN10A) channels1,2.
Target Various KV channels
Peptide Content: 100%
Lyophilized Powder
Guangxitoxin-1E (#STG-200) is a highly pure, synthetic, and biologically active peptide toxin.
Image & Title 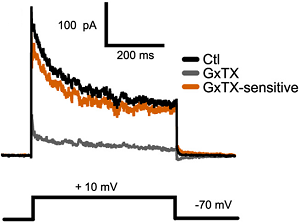
Alomone Labs Guangxitoxin-1E blocks KV2 channels in mouse acute cortical brain slices.KV2 mediated currents in mouse pyramidal cells are blocked by 100 nM application of Guangxitoxin-1E (#STG-200) (grey trace). Voltage protocol is shown below.Adapted from Bishop, H.I. et al. (2015) J. Neurosci. 35, 14922. with permission of the Society for Neuroscience.

Alomone Labs Guangxitoxin-1E blocks KV2 channels in mouse acute cortical brain slices.KV2 mediated currents in mouse pyramidal cells are blocked by 100 nM application of Guangxitoxin-1E (#STG-200) (grey trace). Voltage protocol is shown below.Adapted from Bishop, H.I. et al. (2015) J. Neurosci. 35, 14922. with permission of the Society for Neuroscience.
For research purposes only, not for human use
Last Update: 07/05/2024
Applications
Citations
Citations
Product citations
- Gayet-Primo, J. et al. (2018) J. Neurosci. 38, 3414.
- Smith, P. et al. (2018) Sci. Rep. 8, 7664.
- Fletcher, E.V. et al. (2017) Nat. Neurosci. 20, 905.
- Honigsperger, C. et al. (2017) J. Physiol. 595, 739.
- Frazzini, V. et al. (2016) Cell Death Dis. 7, 22100.
- Pathak, D. et al. (2016) J. Neurophysiol. 115, 2317.
- Stas, J.I. et al. (2016) Sci. Rep. 6, 35080.
- Bishop, H.I. et al. (2015) J. Neurosci. 35, 14922.

