Overview
- Peptide (C)KQLGPGKKNDEVS, corresponding to amino acid residues 151-163 of rat EAAT2 (Accession P31596). Extracellular, 2nd loop.

 Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:800).
Western blot analysis of rat brain membranes (lanes 1 and 3) and mouse brain lysates (lanes 2 and 4):1-2. Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:800).
3-4. Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody, preincubated with EAAT2 (GLT-1) (extracellular) Blocking Peptide (BLP-GC022).
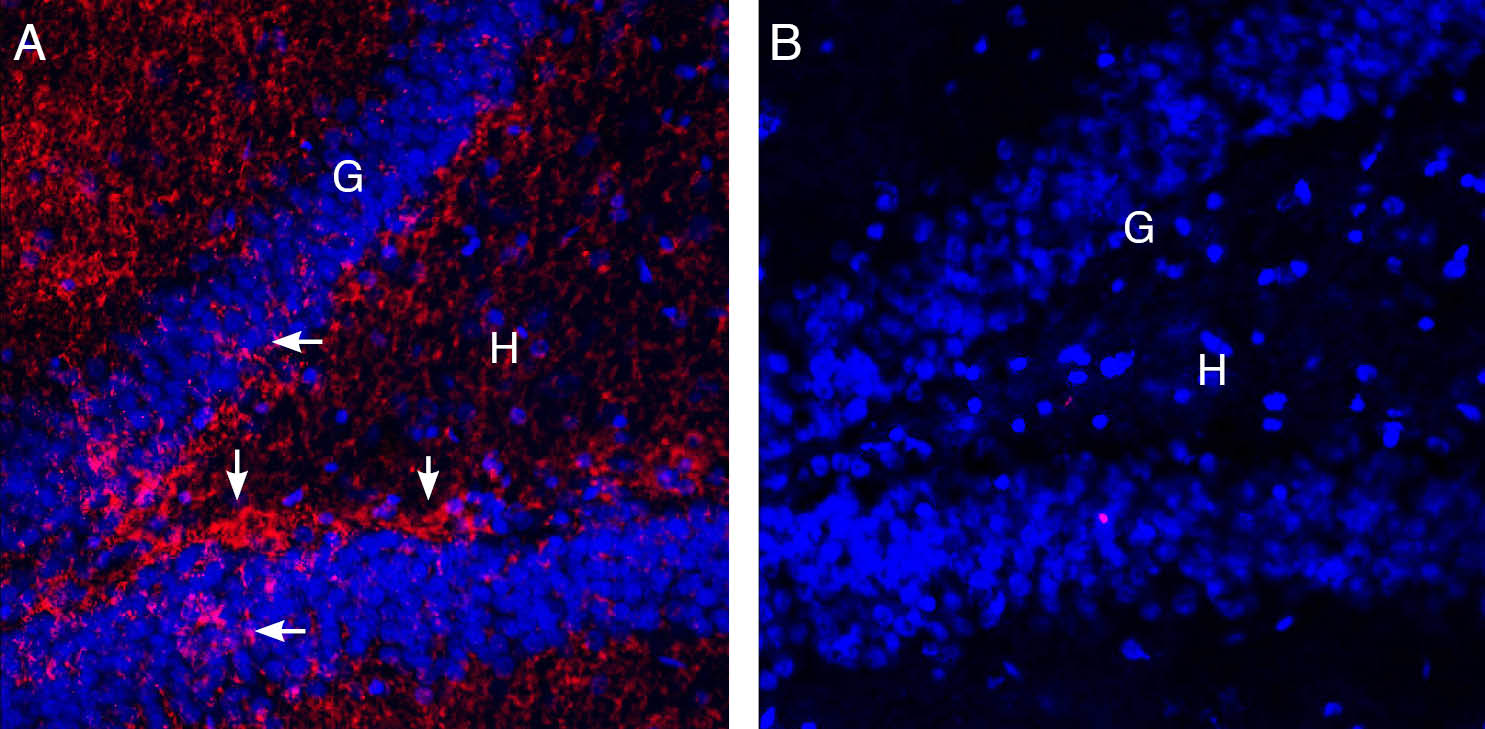 Expression of EAAT2 in rat hippocampus.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:100), followed by goat anti-guinea pig-AlexaFluor-594. A. Staining in the hippocampal dentate gyrus region, showed immunoreactivity (red) in the hilus (H) subgranular layer (vertical arrows) and between cells in the granule layer (G) (horizontal arrows). B. Pre-incubation of the antibody with EAAT2 (GLT-1) (extracellular) Blocking Peptide (BLP-GC022), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of EAAT2 in rat hippocampus.Immunohistochemical staining of perfusion-fixed frozen rat brain sections with Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:100), followed by goat anti-guinea pig-AlexaFluor-594. A. Staining in the hippocampal dentate gyrus region, showed immunoreactivity (red) in the hilus (H) subgranular layer (vertical arrows) and between cells in the granule layer (G) (horizontal arrows). B. Pre-incubation of the antibody with EAAT2 (GLT-1) (extracellular) Blocking Peptide (BLP-GC022), suppressed staining. Cell nuclei are stained with DAPI (blue). Expression of EAAT2 in mouse hippocampus.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:100), followed by goat anti-guinea pig-AlexaFluor-594. A. Staining in the hippocampal dentate gyrus region, showed immunoreactivity (red) in the hilus (H) subgranular layer (vertical arrows) and between cells in the granule layer (G) (horizontal arrows). B. Pre-incubation of the antibody with EAAT2 (GLT-1) (extracellular) Blocking Peptide (BLP-GC022), suppressed staining. Cell nuclei are stained with DAPI (blue).
Expression of EAAT2 in mouse hippocampus.Immunohistochemical staining of perfusion-fixed frozen mouse brain sections with Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP), (1:100), followed by goat anti-guinea pig-AlexaFluor-594. A. Staining in the hippocampal dentate gyrus region, showed immunoreactivity (red) in the hilus (H) subgranular layer (vertical arrows) and between cells in the granule layer (G) (horizontal arrows). B. Pre-incubation of the antibody with EAAT2 (GLT-1) (extracellular) Blocking Peptide (BLP-GC022), suppressed staining. Cell nuclei are stained with DAPI (blue).
- Tzingounis, A.V. and Wadiche, J.I. (2007) Nat. Rev. Neurosci. 8, 935.
- Beart, P.M. and O’Shea, R.D. (2007) Br. J. Pharmacol. 150, 5.
L-Glutamate (Glu) is an abundant amino acid that functions as the major excitatory neurotransmitter in the central nervous system. However, excess of Glu in the extracellular synaptic milieu leads to neuronal cell death by a process known as excitotoxicity.
The extracellular levels of Glu are regulated by a family of high affinity plasma membrane transporters called excitatory amino acid transporters (EAATs) which are responsible for the re-uptake of Glu into the cells1,2.
The EAAT family includes five members (EAAT1-EAAT5) that are members of the solute carrier family 1 (SLC1) of Na+-dependent transporters that also includes the neutral amino acid transporters ASCT1 and ASCT2.
The Glu transporters present an unusual topology of eight transmembrane domains with two re-entrant loops and intracellular N- and C- termini. The transporter is likely assembled as a trimer where each monomer is a functional unit capable of binding the Glu substrate.
The transport of Glu into the cells by the EAAT transporters is coupled to the Na+ and K+ electrochemical gradient as a driving force. Hence, the uptake of Glu is dependent on the co-transport of three Na+ and one H+ ions, and the counter transport of one K+ ion.
In addition, to the well documented Glu uptake, the EAAT transporters show a Glu-independent Cl- conductance. The physiological significance of the Cl- current through the EAATs is currently unknown1,2.
EAAT2 (also known as Glutamate Transporter-1, GLT-1) as well as EAAT1, is expressed predominantly in glia cells, while EAAT3, EAAT4 and EAAT5 are mostly expressed in neurons.
As mentioned earlier, EAAT transporters represent the only (significant) mechanism for removal of glutamate from the extracellular fluid and hence are essential for the long-term maintenance of low and non-toxic concentrations of glutamate and the preservation of normal excitatory synaptic transmission.
In addition to Glu uptake, the glutamate transporters provide glutamate for the synthesis of γ-Aminobutyric acid (GABA), glutathione and protein, suggesting an interactive role between EAATs and cellular metabolism1,2.
Dysregulation of EAAT activities has been implicated in several neurodegenerative disorders such as Alzheimer’s disease, traumatic brain injury, epilepsy and schizophrenia, suggesting that EAATs can be a useful target for the treatment of these conditions1,2.
Application key:
Species reactivity key:
Guinea Pig Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022-GP, formerly AGP-113) is a highly specific antibody directed against an extracellular epitope of the rat EAAT2 protein. The antibody can be used in western blot analysis and immunohistochemistry applications. It has been designed to recognize EAAT2 (GLT-1) from rat, mouse and human samples.
The antigen used to immunize guinea pigs is the same as Anti-EAAT2 (GLT-1) (extracellular) Antibody (#AGC-022) raised in rabbit. Our line of guinea pig antibodies enables more flexibility with our products such as multiplex staining studies, immunoprecipitation and more.
