Overview
- Peptide (C)EKPFHLNYHVDHLD, corresponding to amino acid residues 60-73 of rat GluR3 (Accession P19492). Extracellular, N-terminus.

 Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain membranes, human SH-SY5Y neuroblastoma cell line (lanes 5 and 7) and human Jurkat T-cell leukemia cell line (lanes 6 and 8) lysates:1,2. Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010-GP) (1:2000).
Western blot analysis of rat (lanes 1 and 3) and mouse (lanes 2 and 4) brain membranes, human SH-SY5Y neuroblastoma cell line (lanes 5 and 7) and human Jurkat T-cell leukemia cell line (lanes 6 and 8) lysates:1,2. Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010-GP) (1:2000).
5,6. Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (1:400).
3,4,7,8. Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody, preincubated with GluR3/GluA3 (extracellular) Blocking Peptide (#BLP-GC010)
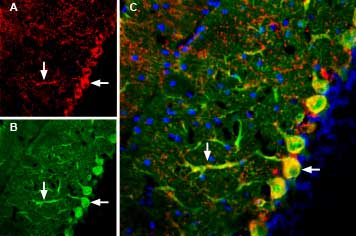 Expression of GluR3 (GluA3) in mouse brainImmunohistochemical staining of mouse brain sections using Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010-GP). A. GluA3 immunoreactivity (red) in the cerebellum appears in Purkinje cell soma (horizontal arrow) and dendrites (vertical arrow). B. Parvalbumin staining (green) appears in Purkinje cells. C. Merged image of panels A and B demonstrates partial colocalization some Purkinje soma and dendrites (arrows). Nuclei are demonstrated using DAPI as the counterstain (blue).
Expression of GluR3 (GluA3) in mouse brainImmunohistochemical staining of mouse brain sections using Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010-GP). A. GluA3 immunoreactivity (red) in the cerebellum appears in Purkinje cell soma (horizontal arrow) and dendrites (vertical arrow). B. Parvalbumin staining (green) appears in Purkinje cells. C. Merged image of panels A and B demonstrates partial colocalization some Purkinje soma and dendrites (arrows). Nuclei are demonstrated using DAPI as the counterstain (blue).
- Dingledine, R. et al. (1999) Pharmacol. Rev. 51, 7.
- Lynch, M.A. (2004) Physiol. Rev. 84, 87.
- Steenland, H.W. et al. (2008) Eur. J. Neurosci. 27, 1166.
L-Glutamate, the major excitatory neurotransmitter in the central nervous system, operates through several receptors that are categorized as ionotropic (ligand-gated cation channels) or metabotropic (G-protein coupled receptors).
The ligand-gated ion channel family consists of 15 members that have been subdivided into three families based on their pharmacological profile: a-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA), and the kainate receptors.
The AMPA receptor subfamily includes four members AMPA1 to AMPA4, also known as GluR1 to GluR4 respectively.
The functional AMPA channel is believed to be a tetramer, with most neuronal AMPA receptors being heterotetramers composed of AMPA1/AMPA2 or AMPA2/AMPA3 channels, although homotetramers can also been found.
AMPA receptors are permeable to cations Na+, K+ and Ca2+. The Ca2+ permeability is dependent on the presence of AMPA21.
Gating of AMPA receptors by glutamate is extremely fast and therefore the AMPA receptors mediate most excitatory (depolarizing) currents in the brain during basal neuronal activity. The depolarization caused by the activation of post-synaptic AMPA receptors is necessary for the activation of NMDA receptors that will open only in the presence of both glutamate and a depolarized membrane potential.
Synaptic strength that is defined as the level of post-synaptic depolarization can be long term (hence the term long term potentiation, LTP) and therefore induce changes in signaling and protein synthesis in the activated neuron. These changes are associated with memory formation and learning. Changes in synaptic strength are thought to involve rapid movement of the AMPA receptors in and out of the synapses. A great deal of effort is focused on understanding the mechanisms that govern AMPA receptor trafficking2.
The exact physiological role of the AMPA3 receptor is not clear but a role in the modulation of oscillatory networks affecting sleep and breathing has been suggested3.
Application key:
Species reactivity key:
Guinea pig Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010-GP, formerly AGP-142), raised in guinea pigs, is a highly specific antibody directed against an epitope of the rat protein. The antibody can be used in western blot and immunohistochemistry applications. It has been designed to recognize GluR3 from mouse, rat, and human samples. The antigen used to immunize guinea pigs is the same as Anti-GluR3 (GluA3) (extracellular) Antibody (#AGC-010) raised in rabbit. Our line of guinea pig antibodies enables more flexibility with our products such as multiplex staining studies, immunoprecipitation, etc.
