Overview
- Lebrun, B. et al. (1997) Biochem. J. 328, 321.
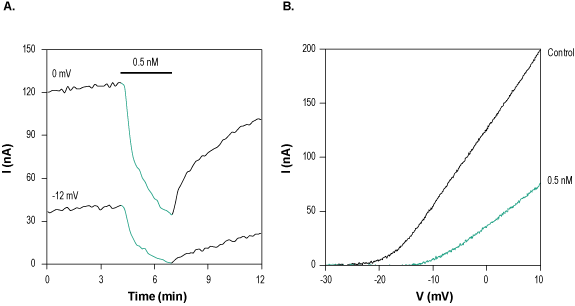 Alomone Labs HsTx-1 Toxin inhibits KV1.3 channels heterologously expressed in Xenopus oocytes.A. Time course of HsTx1 Toxin (#STH-300) action on KV1.3 currents. Current amplitudes were plotted as a function of time. Membrane potential was held at -80 mV and oocytes were stimulated by a 100 ms voltage ramp to +10 mV. 0.5 nM HsTx1 Toxin was perfused during 160 sec, as indicated (green) at 0 mV (70% inhibition) and -12 mV (95% inhibition). B. Superimposed examples of KV1.3 channel current in the absence (black) and presence (green) of 0.5 nM HsTx1 Toxin (taken from the experiment in A).
Alomone Labs HsTx-1 Toxin inhibits KV1.3 channels heterologously expressed in Xenopus oocytes.A. Time course of HsTx1 Toxin (#STH-300) action on KV1.3 currents. Current amplitudes were plotted as a function of time. Membrane potential was held at -80 mV and oocytes were stimulated by a 100 ms voltage ramp to +10 mV. 0.5 nM HsTx1 Toxin was perfused during 160 sec, as indicated (green) at 0 mV (70% inhibition) and -12 mV (95% inhibition). B. Superimposed examples of KV1.3 channel current in the absence (black) and presence (green) of 0.5 nM HsTx1 Toxin (taken from the experiment in A).
- Miller, C. et al. (1995) Neuron 15, 5.
- Bontems, F. et al. (1991) Science 254, 1521.
- Martinez, F. et al. (1998) FEBS Lett 429, 381.
- Lebrun, B. et al. (1997) Biochem. J. 328, 321.
- Kharrat, R. et al. (1996) Eur. J. Biochem. 242, 491.
Many K+ channel blockers have been identified in the scorpion venom1. These peptide toxins consist of 29 to 39 amino acids, and commonly share three disulfide bonds. Their 3D structure comprises a helix and a double- or triple-stranded β-sheet2. The functional sites of the toxins are located either on the solvent exposed face of the β-sheet, or on the helix, depending on the type of K+ channel targeted3.
Potassium channel toxin alpha-KTx 6.3 (HsTx1) is a 34 amino acid peptidyl toxin isolated from the Heterometrus spinifer (Asia giant forest scorpion, Malaysian black scorpion) venom4. HsTx1 is one of the most active peptidic inhibitors of rat KV1.3 channels ever described, with an IC50 of approx. 12 pM4. HsTX1 belongs to a structural class of scorpion K+ channel inhibitors which includes Pandinus imperator toxin 1 (Pi1) and Maurotoxin (MTX) (#STM-340), two toxins containing 35 residues and four disulphide bridges5.
HsTx1 Toxin (#STH-300) is a highly pure, synthetic, and biologically active peptide toxin.

