Overview
- Tolkovsky, A. (1997) Trends Neurosci. 20, 1.
- Jing, S. et al. (1992) Neuron 9, 1067.
- Acheson, A. et al. (1995) Nature 374, 450.
- Morse, J.K. et al. (1993) J. Neurosci. 13, 4146.
- Hyman, C. et al. (1991) Nature 350, 230.
- Friedman, B. et al. (1995) J. Neurosci. 15, 1044.
- Meyer, M. et al. (1992) J. Cell Biol. 119, 45.
- Koliatsos, V.E. et al. (1993) Neuron 10, 359.
- Teng, H.K. et al. (2005) J. Neurosci. 25, 5455.
- Woo, N.H. et al. (2005) Nat. Neurosci. 8, 1069.
 Alomone Labs Recombinant human proBDNF protein mediates neurite outgrowth in TrkB transfected PC12 cells.Cells were transiently transfected with TrkB/pcDNA3 containing the green fluorescence protein (GFP) as a reporter. One day post transfection, the cells were stimulated with 20 ng/ml Recombinant human proBDNF protein (#B-257) or 10ng/ml Recombinant human BDNF protein (#B-250). Development of neurites was visualized after 6 days using bright light microscopy.
Alomone Labs Recombinant human proBDNF protein mediates neurite outgrowth in TrkB transfected PC12 cells.Cells were transiently transfected with TrkB/pcDNA3 containing the green fluorescence protein (GFP) as a reporter. One day post transfection, the cells were stimulated with 20 ng/ml Recombinant human proBDNF protein (#B-257) or 10ng/ml Recombinant human BDNF protein (#B-250). Development of neurites was visualized after 6 days using bright light microscopy.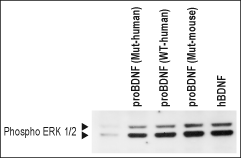 Alomone Labs Recombinant human proBDNF protein mediates ERK1/2 activation in TrkB transfected HEK293 cells.Transfected cells were serum depleted for 2 h and then challenged with or without Recombinant human proBDNF (cleavage resistant) protein (#B-256), Recombinant human proBDNF protein (#B-257), Recombinant mouse proBDNF (cleavage resistant) protein (#B-243) or Recombinant human BDNF protein (#B-250) for 10 min. Cell proteins were resolved by SDS-PAGE and detected with anti-phospho-ERK1/2.
Alomone Labs Recombinant human proBDNF protein mediates ERK1/2 activation in TrkB transfected HEK293 cells.Transfected cells were serum depleted for 2 h and then challenged with or without Recombinant human proBDNF (cleavage resistant) protein (#B-256), Recombinant human proBDNF protein (#B-257), Recombinant mouse proBDNF (cleavage resistant) protein (#B-243) or Recombinant human BDNF protein (#B-250) for 10 min. Cell proteins were resolved by SDS-PAGE and detected with anti-phospho-ERK1/2.
- Bibel, M. and Barde, Y.A. (2000) Genes Dev. 14, 2929.
- Cahoon-Metzger, S.M. et al. (2001) Dev. Biol. 232, 246.
- Troy, C.M. et al. (2002) J. Biol. Chem. 277, 34295.
- Chen, Z.Y. et al. (2004) J. Neurosci. 24, 4401.
- Egan, M.F. et al. (2003) Cell 112, 257.
- Lee, R. et al. (2001) Science 294, 1945.
- Teng, H.K. et al. (2005) J. Neurosci. 25, 5455.
- Woo, N.H. et al. (2005) Nat. Neurosci. 8, 1069.
- Fayard, B. et al. (2005) J. Neurosci. Res. 80, 18.
- Michalski, B. and Fahnestock, M. (2003) Mol. Brain Res. 111, 148.
- Zhou, X.F. et al. (2004) J. Neurochem. 91, 704.
BDNF is a neurotrophic factor produced by proteolytic cleavage of its precursor, proBDNF. The biologically relevant form of the protein was thought to be the mature form, BDNF.1 The actions of BDNF are mediated via the binding to TrkB or p75.2,3
The precursor form was thought to be important for the correct folding, secretion and trafficking of the mature protein. A single-nucleotide polymorphism (Val66 to Met) in the pro-domain of the human BDNF gene impairs intracellular trafficking and regulated secretion of BDNF in primary cortical neurons and neurosecretory cells but not in endothelial and vascular cells.4 This has been shown to affect memory and lead to abnormal hippocampal function in humans.5
The finding that proBDNF and not mature BDNF is the preferred ligand for p75, has ushered in a new era which reexamines the biological roles of the two forms.6 Some biological roles for proBDNF have been proposed. It has been shown to be a pro-apoptotic ligand for sympathetic neurons7 expressing both p75 and sortlin, and to be involved in LTD8. On the other hand, it has also been shown to elicit prototypical TrkB responses in biological assays, such as TrkB tyrosine phosphorylation, and activation of ERK1/2.9
In brain homogenates a mixture of both, proBDNF and mature BDNF has been found10,11 and in cortical neurons secretion of proBDNF has been shown.7 Binding of both proBDNF and mature BDNF to TrkB has been proposed to be via the R103 residue in the mature portion.9
Recombinant human proBDNF protein (#B-257) is a highly pure, recombinant, and biologically active protein.
Applications
Citations
- de la Cruz-Morcillo, M.A. et al. (2016) Oncotarget 7, 34480.
- Luo, C. et al. (2016) Sci. Rep. 6, 27171.
- Hane, M. et al. (2015) Glycobiology 25, 1112.
- Suzuki, R. et al. (2014) J. Neurosci. 34, 3429.
- Bachis, A. et al. (2012) J. Neurosci. 32, 9477.

