Overview
- Tolkovsky, A. (1997) Trends Neurosi. 20, 1.
- Jing, S. et al. (1992) Neuron 9, 1067.
- Acheson, A. et al. (1995) Nature 374, 450.
- Morse, J.K. et al. (1993) J. Neurosci. 13, 4146.
- Hyman, C. et al. (1991) Nature 350, 230.
- Friedman, B. et al. (1995) J. Neurosci. 15, 1044.
- Meyer, M. et al. (1992) J. Cell Biol. 119, 45.
- Koliatsos, V.E. et al. (1993) Neuron 10, 359.
- Teng, H.K. et al. (2005) J. Neurosci. 25, 5455.
- Woo, N.H. et al. (2005) Nat. Neurosci. 8, 1069.
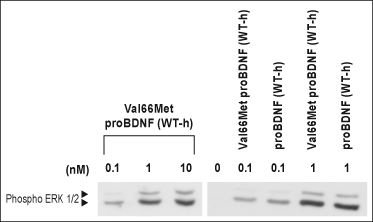 Alomone Labs Recombinant human Val66Met proBDNF protein mediates ERK1/2 phosphorylation as Recombinant human proBDNF protein in TrkB transfected HEK293 cells.Transfected HEK293 cells were serum depleted for 2 hr and then challenged with or without Recombinant human Val66Met proBDNF protein (#B-457) or Recombinant human proBDNF protein (#B-257) for 10 min. The cell proteins were resolved by SDS-PAGE and detected with anti-phospho-ERK1/2 antibody.
Alomone Labs Recombinant human Val66Met proBDNF protein mediates ERK1/2 phosphorylation as Recombinant human proBDNF protein in TrkB transfected HEK293 cells.Transfected HEK293 cells were serum depleted for 2 hr and then challenged with or without Recombinant human Val66Met proBDNF protein (#B-457) or Recombinant human proBDNF protein (#B-257) for 10 min. The cell proteins were resolved by SDS-PAGE and detected with anti-phospho-ERK1/2 antibody.
Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that bind p75NTR as well as TrkB receptors1,2. BDNF supports the survival of many cell types3-8 and also modulates hippocampal plasticity and hippocampal-dependent memory in cell models and in animals9.
The BDNF gene, like other peptide growth factors, encodes a precursor peptide (proBDNF), which is proteolytically cleaved to form the mature protein10. proBDNF has been shown to be a pro-apoptotic ligand for sympathetic neurons expressing both p75NTR and sortilin11, and to be involved in long-term potentiation (LTP) stage of the memory-related modifications in synaptic transmission12.
A nonconservative single nucleotide polymorphism (SNP) in the human BDNF gene has been identified at nucleotide 196 (G/A) producing an amino acid substitution (Valine to Methionine) at codon 66 (Val66Met, rs 6265). Although located in the 5' pro-BDNF region, this SNP resulted in striking deficits in the cellular distribution and regulated secretion of the mature protein, BDNF and hence in corresponding alterations of human hippocampal function and episodic memory in vivo9.
Egan M.F. et al demonstrated the molecular mechanisms that control activity-dependent BDNF secretion and showed that depolarization-dependent secretion of BDNF in hippocampal neurons is significantly impaired when this Val66Met SNP occurs. Using double-staining techniques, they demonstrated that Val-BDNF-containing secretory granules are colocalized with synaptophysin, a marker for synapses. In contrast, Val66Met-BDNF aggregates are accumulated in the cell body and rarely colocalize with synaptophysin. This suggests that even if it can be secreted in small amounts near the cell body through the constitutive pathway, the Met-BDNF protein cannot be secreted at synapses9. Studies of heterozygote BDNF knockout rodents, who presumably have intermediate BDNF levels, demonstrate clear physiological13 and behavioral14 abnormalities, suggesting that secretion levels are critical.
Multiple studies over the recent decades in humans, in vivo in animal models and in vitro found an association between the Val66Met polymorphism and bipolar and unipolar disorders15, Schizophrenia16, 17, anxiety-related behavior18,19 and controversial association with ADHD20,21.
The data emerged from the analysis of the Val66Met phenotype in various syndromes and diseases reveal the importance of the pro-region of the BDNF polypeptide, particular Valine66 and perhaps the nearby sequence, in intracellular trafficking and secretion of BDNF.
