Overview
- Wang, M. et al. (2008) Biochem. Pharmacol. 76, 1716.
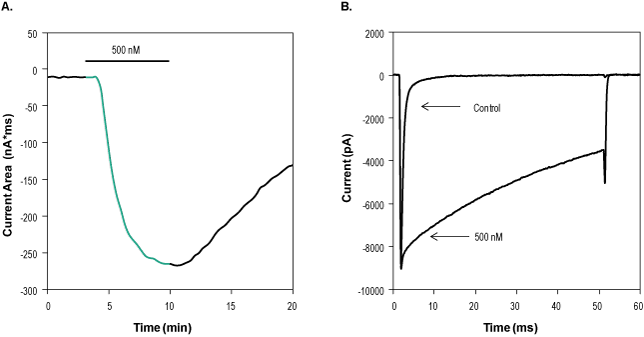 Alomone Labs Jingzhaotoxin-II activates NaV1.5 currents in stably transfected HEK293 cells.A. Time course of Jingzhaotoxin-II (#STJ-150) action. Current area was plotted as a function of time. Holding potential was -100 mV and currents were stimulated every 20 seconds by a voltage step of 50 msec from holding potential to -20 mV. 500 nM Jingzhaotoxin-II was perfused in the period marked by the horizontal bar (green), indicating a toxin-dependent decrease in NaV1.5 currents inactivation. B. Superimposed traces of NaV1.5 currents before and during 7 min application of 500 nM Jingzhaotoxin-II.
Alomone Labs Jingzhaotoxin-II activates NaV1.5 currents in stably transfected HEK293 cells.A. Time course of Jingzhaotoxin-II (#STJ-150) action. Current area was plotted as a function of time. Holding potential was -100 mV and currents were stimulated every 20 seconds by a voltage step of 50 msec from holding potential to -20 mV. 500 nM Jingzhaotoxin-II was perfused in the period marked by the horizontal bar (green), indicating a toxin-dependent decrease in NaV1.5 currents inactivation. B. Superimposed traces of NaV1.5 currents before and during 7 min application of 500 nM Jingzhaotoxin-II.
Voltage-gated Na+ channels play a very important role in the upstroke of action potentials, forming the basis for electrical signaling in the nervous system, and control the flow of ions across plasma membrane in response to changes in voltage1. In vertebrates, nine different pore-forming Na+ channels α subunits have been characterized and cloned in cardiac myocytes, neurons, and skeletal muscle. These Na+ channel isoforms are often classified as TTX-sensitive (NaV1.1–1.4, NaV1.6, and NaV1.7) and TTX-resistant (NaV1.5, NaV1.8, and NaV1.9)2.
NaV1.5, the major cardiac voltage-gated Na+ channel, plays a central role in the generation of the cardiac action potential and in the propagation of electrical impulses in the heart3.
Jingzhaotoxin-II, a 32-residue polypeptide, isolated from the venom of Chinese tarantula Chilobrachys jingzhao. JZTX-II has a high affinity for the tetrodotoxin-resistant (TTX-R) voltage-gated Na+ channels in cardiac myocytes. It significantly slows rapid inactivation with an IC50 value of 260 nM. Although JZTX-II does not have an effect on TTX-R neuronal channels in DRG neurons it does affect TTX-sensitive Na+ currents by slowing down their inactivation4.

