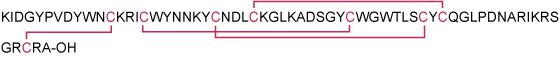Overview
Synthetic peptide
- Chuang, R.S. et al. (1998) Nat. Neurosci. 1, 668.
- Lee, C.W. et al. (2011) Biochem. Biophys. Res. Commun. 416, 277.
 Alomone Labs Kurtoxin blocks CaV3.1 channels expressed in Xenopus oocytes.A. Representative time course of Kurtoxin (#STK-800) inhibition of CaV3.1 current. Membrane potential was held at -100 mV, current was elicited by a 100 ms voltage step to -30 mV every 10 sec, and significantly inhibited by application of 50 nM Kurtoxin (green). B. Superimposed traces of CaV3.1 current following the application of control (black) and of 50 nM Kurtoxin (green), taken from the recording in A.
Alomone Labs Kurtoxin blocks CaV3.1 channels expressed in Xenopus oocytes.A. Representative time course of Kurtoxin (#STK-800) inhibition of CaV3.1 current. Membrane potential was held at -100 mV, current was elicited by a 100 ms voltage step to -30 mV every 10 sec, and significantly inhibited by application of 50 nM Kurtoxin (green). B. Superimposed traces of CaV3.1 current following the application of control (black) and of 50 nM Kurtoxin (green), taken from the recording in A.
Kurtoxin is a peptide toxin originally isolated from the South African scorpion (Parabuthus transvaalicus) venom, and was the first characterized ligand for low voltage-gated calcium channels1,2. Kurtoxin potently blocks CaV3.1 and CaV3.2 channels displaying effective working concentrations as low as 50 nM.
Although Kurtoxin is very similar to other scorpion isolated toxins (also known as α-toxins) that target sodium channels, detailed NMR analyses uncovered a unique configuration that distinguishes it from α-toxins and, therefore, is believed to be essential for Kurtoxin’s binding ability3.
CaV3.1 and CaV3.2 are members of the T-type calcium channels that regulate calcium intake near resting membrane potential1. Members of the CaV3 ion channels control cardiac pacemaking, but may also play a role outside the nervous system as predicted by their overall distribution in the body1.